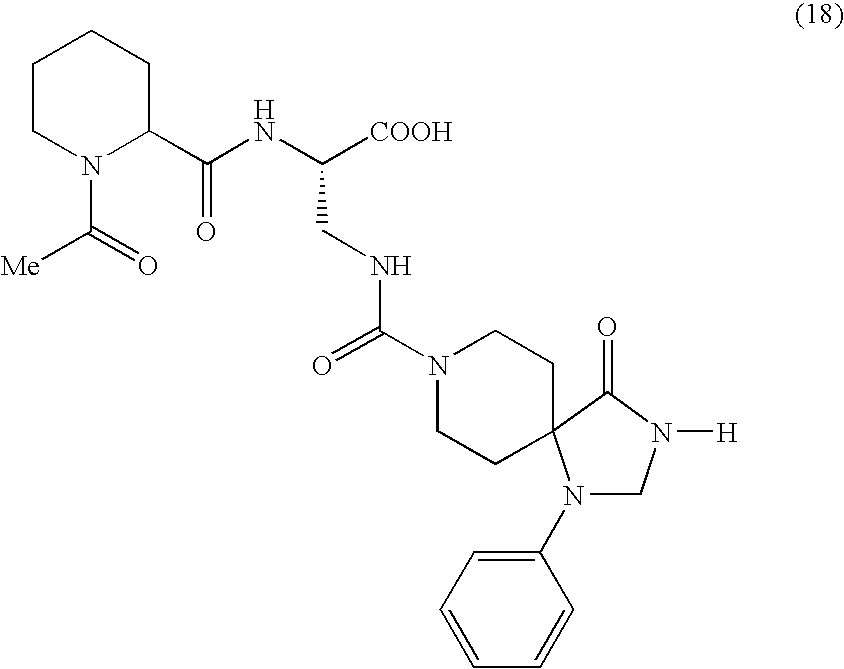
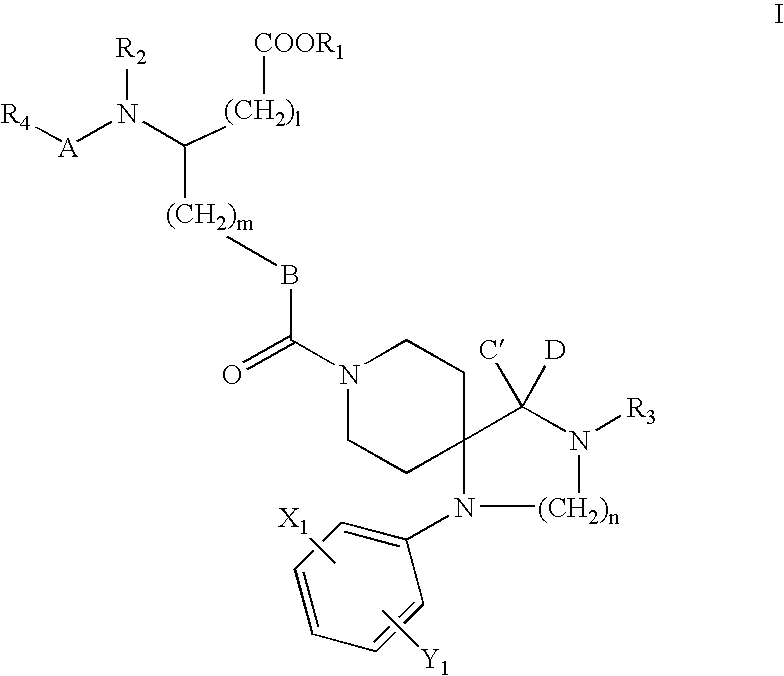
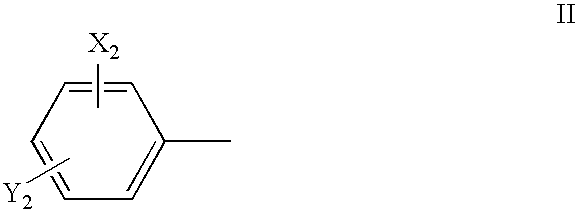Spiro derivatives and adhesion molecule inhibitors comprising the same as active ingredient
a technology of adhesion molecule and active ingredient, which is applied in the direction of peptides, drug compositions, immunological disorders, etc., can solve the problems of achieve the effect of suppressing the activation of leukocytes and not inhibiting the progress of inflammation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Methyl 2-((t-butoxy)carbonylamino)-3-((2,4,8-triaza-2-methyl-1-oxo-4-phenylspiro[4.5]dec-8-yl)carbonylamino)propanoate (1)
[0237]
[0238] Under argon atmosphere, 12.9 g of methyl 3-amino-2-((t-butoxy)carbonylamino)propanoate was dissolved in 700 ml of dichloromethane, and then 7.9 g of saturated sodium hydrogen carbonate and 14.3 g of p-nitrophenyl chloroformate were added thereto at 0° C., followed by stirring the resulting mixture at room temperature for 5.5 hours. To the reaction mixture, 21.7 g of 2,4,8-triaza-2-methyl-4-phenylspiro[4.5]decane-1-one and 41.1 ml of triethylamine were added, and the resulting mixture was stirred at room temperature for 13 hours. After concentrating the reaction mixture, saturated aqueous sodium hydrogen carbonate solution was added, and the resulting mixture was extracted with chloroform. The organic layers were combined, washed with 0.1 N hydrochloric acid and with saturated saline, dried over anhydrous sodium sulfate and concentrated. The residue ...
example 2
Methyl 2-((t-butoxy)carbonylamino)-3-((2,4,8-triaza-1-oxo-4-phenylspiro[4.5]dec-8-yl)carbonylamino)propanoate (2)
[0241]
[0242] Under argon atmosphere, 564 mg of 1,1-carbonyldiimidazole was dissolved in 8 ml of tetrahydrofuran, and into this solution 10 ml of tetrahydrofuran containing 760 mg of methyl 3-amino-2-((t-butoxy)carbonylamino)propanoate was dropped at 0° C. over 25 minutes. The resulting solution was then stirred for 0.5 hours. To the reaction mixture, 805 mg of 2,4,8-triaza-4-phenylspiro[4.5]decane-1-one was added, and the resulting mixture was stirred at room temperature for 13 hours. To the reaction mixture, 10% aqueous citric acid was added, and the resulting mixture was extracted with ethyl acetate. The organic layers were combined, washed with 0.1 N hydrochloric acid and with saturated saline, dried over anhydrous sodium sulfate and concentrated to obtain 1.55 g of methyl 2-((t-butoxy)carbonylamino)-3-((2,4,8-triaza-1-oxo-4-phenylspiro[4.5]dec-8-yl)carbonylamino)prop...
example 3
Methyl 2-((phenylsulfonyl)amino)-3-((2,4,8-triaza-2-methyl-1-oxo-4-phenylspiro[4.5]dec-8-yl)carbonylamino)propanoate (3)
[0245]
[0246] In 2 ml of dichloromethane, 362 mg of methyl 2-((t-butoxy)carbonylamino)-3-((2,4,8-triaza-2-methyl-1-oxo-4-phenylspiro[4.5]dec-8-yl)carbonylamino) propanoate was dissolved, and 1 ml of trifluoroacetic acid was added thereto, followed by stirring the resulting mixture at room temperature for 12 hours. The reaction mixture was concentrated then dissolved in chloroform, washed with aqueous potassium carbonate (0.5 M) and with saturated saline, dried over anhydrous sodium sulfate, and concentrated. The residue was dissolved in 4 ml of dichloromethane, and then 205 μl of triethylamine and 95 μl of benzenesulfonyl chloride were added thereto, followed by stirring the resulting mixture at room temperature overnight. Saturated aqueous sodium hydrogen carbonate solution was added to the reaction mixture, and the resulting mixture was extracted with chloroform....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com