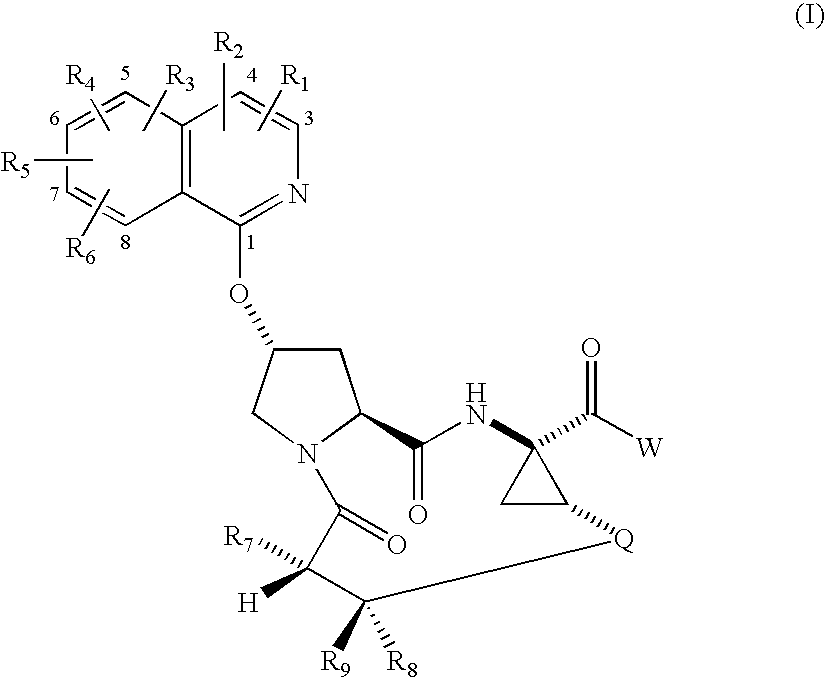
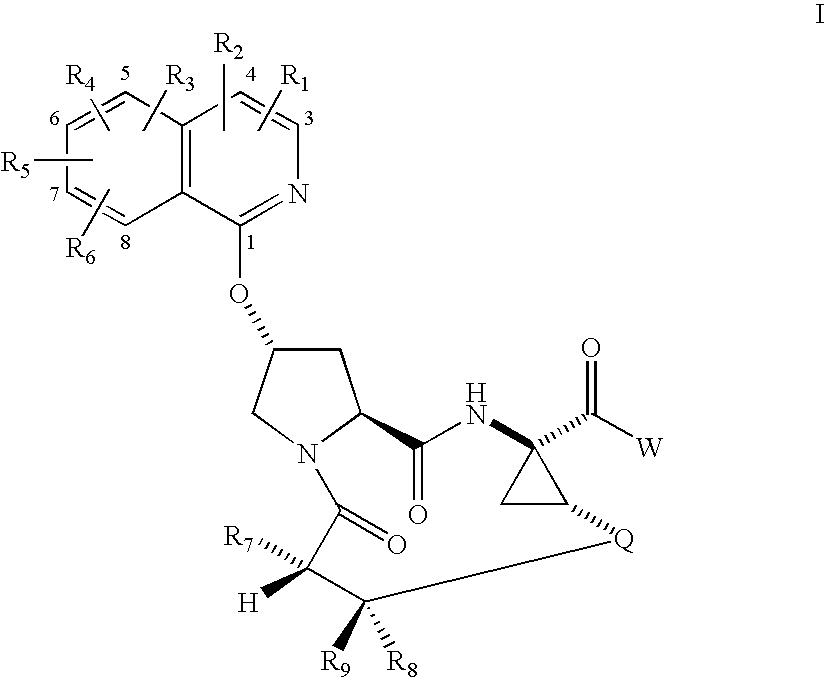
Macrocyclic isoquinoline peptide inhibitors of hepatitis C virus
a technology of macrocyclic isoquinoline and hepatitis c virus, which is applied in the direction of peptides, drug compositions, peptides, etc., can solve the problems that a large percentage of patients do not have a sustained reduction in viral load, and achieve the effect of inhibiting the function of ns3 proteas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Representative Intermediates
Preparation of P2 Isoquinoline Intermediates for Incorporation into Compounds of Formula 1
[0167] Method A
Step 1:
[0168] To a solution of 3-methoxy cinnamic acid (11.04 g, 62 mmol) and triethylamine (12.52 g, 124 mmol) in acetone (80 mL) was added ethyl chloroformate (approximately 1.5 equivalents) dropwise at 0° C. After stirring at this temperature for 1 h, aqueous NaN3 (6.40 g, 100 mmol in 35 mL H2O; appropriate precautions must be taken when using sodium azide) was added dropwise and the reaction mixture was stirred for 16 h at the ambient temperature. Water (100 mL) was added to the mixture and the volatile was removed in vacuo. The resulting slurry was extracted with toluene (3×50 mL) and the combined organic layers were dried over MgSO4. This dried solution was added dropwise to a heated solution of diphenylmethane (50 mL) and tributylamine (30 mL) at 190° C. The toluene was distilled off as added. After complete addition, the r...
example 2
, Compound 1
Step 2A, Preparation of 2-(1-Ethoxycarbonyl-2-vinyl-cyclopropylcarbamoyl)-4-(isoquinolin-1-yloxy)-pyrrolidine-1-carboxylic acid tert-butyl ester
[0329]
[0330] A stirred slurry of 4-(isoquinolin-1-yloxy)-pyrrolidine-1,2-dicarboxylic acid 1-tert-butyl ester (5.69 g, 15.9 mmol) in 200 mL of methylene chloride was treated sequentially with diisopropylethylamine (13.8 mL, 79.0 mmol), HBTU (7.10 g, 18.7 mmol), HOBT.H2O (2.86 g, 18.7 mmol), and 1R,2S-1-amino-2-vinylcyclopropane carboxylic acid ethyl ester hydrochloride (3.19 g, 16.7 mmol). The gold homogeneous solution was stirred at rt under N2 for 18 h, and then concentrated in vacuo to give 10 g of a brown oil. This was partitioned between ethyl acetate and sat. aq. NaHCO3. The organic phase was washed with brine, dried (MgSO4), and concentrated in vacuo. Flash chromatography (2-5% MeOH in methylene chloride) gave 5.5 g (70%) of 2-(1-ethoxycarbonyl-2-vinyl-cyclopropylcarbamoyl)-4-(isoquinolin-1-yloxy)-pyrrolidine-1-carboxylic...
example 3a
Preparation of Cyclopropylsulfonamide
[0339] Method A:
[0340] To a solution of 100 mL of THF cooled to 0° C. was bubbled in gaseous ammonia until saturation was reached. To this solution was added a solution of 5 g (28.45 mmol) of cyclopropylsulfonyl chloride (purchased from Array Biopharma) in 50 mL of THF, the solution warmed to rt overnite and stirred one additional day. The mixture was concentrated until 1-2 mL of solvent remained, applied on to 30 g plug of SiO2 (eluted with 30% to 60% EtOAc / Hexanes) to afford 3.45 g (100%) of cyclopropyl sulfonamide as a white solid. 1H NMR (Methanol-d4) δ 0.94-1.07 (m, 4H), 2.52-2.60 (m, 1H); 13C NMR (methanol-d4) δ 5.92, 33.01.
Method B:
Step 1: Preparation of N-tert-Butyl-(3-chloro)propylsulfonamide
[0341]
tert-Butylamine (3.0 mol, 315.3 mL) was dissolved in THF (2.5 L). The solution was cooled to −20° C. 3-Chloropropanesulfonyl chloride (1.5 mol, 182.4 mL) was added slowly. The reaction mixture was allowed to warm to rt and stirred for 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com