Desulfurization system and method for desulfurizing a fuel stream
a technology of desulfurization system and fuel stream, which is applied in the direction of filtration separation, multi-stage water/sewage treatment, separation process, etc., can solve the problems of shortening the life expectancy of the components of the fuel cell processing train, and affecting the efficiency of the desulfurization system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
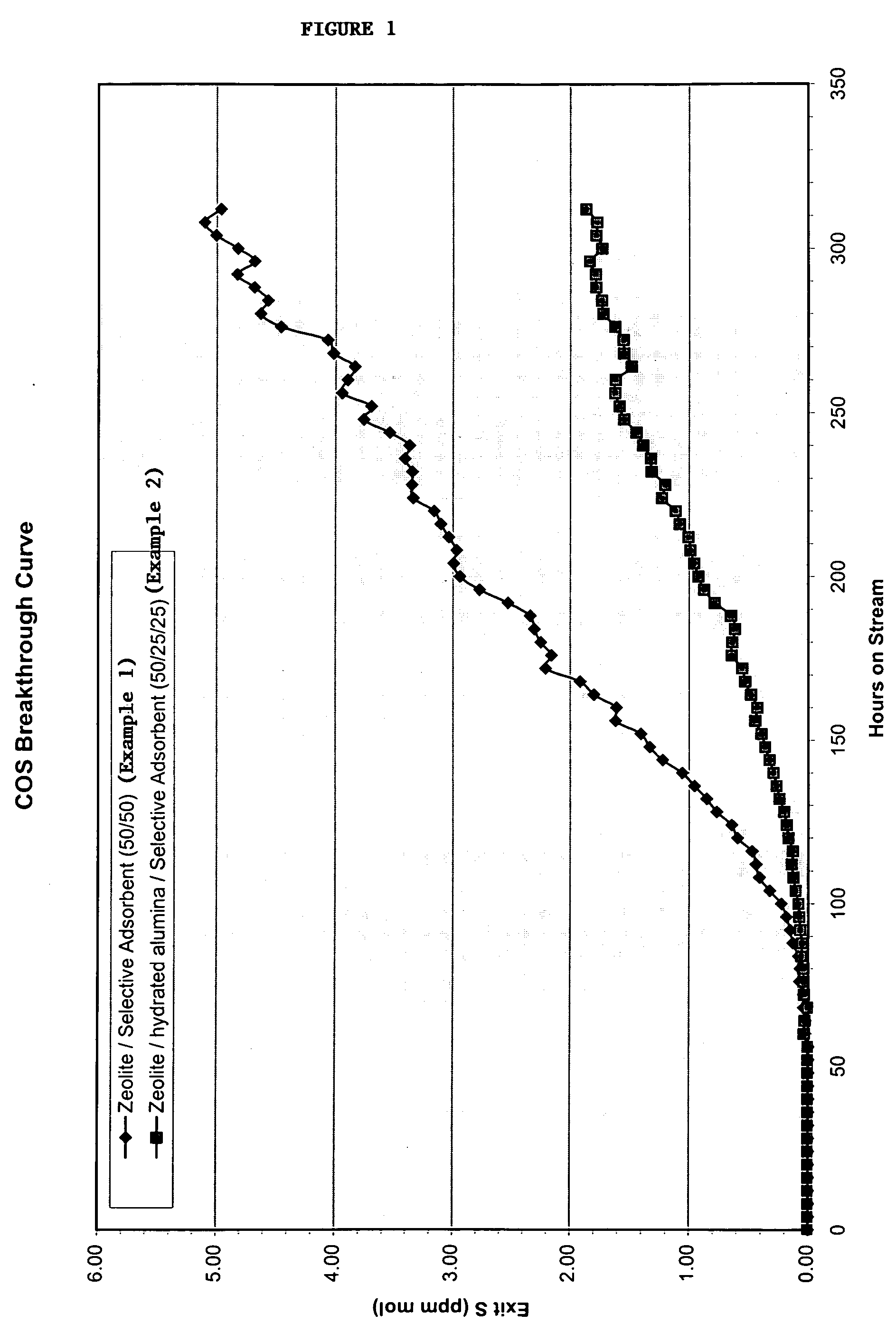
[0073] The synthetic natural gas containing carbonyl sulfide is passed through a reactor containing, in sequence, calcium-exchanged zeolite X and the selective sulfur adsorbent. The zeolite X has an Si:Al equivalent ratio of 1.17 and a calcium exchange of 70% with the remaining metal ions comprising sodium and / or potassium. The selective sulfur adsorbent comprises 70% by weight manganese compounds, 21% copper oxide comprising Cuo and 9% silica. The temperature of the reactor is maintained at 38° C. and the pressure is maintained at about 2 bar. The sulfur adsorbency of the two component system is shown on FIG. 1, and shows breakthrough occurring at 136 hours. The percent S in the form of COS that is removed by this system is listed in FIG. 2.
example 2
[0074] A further test is run wherein the calcium exchanged zeolite and selective sulfur adsorbent of Example 1 are used in combination with a hydrated alumina in the reactor. The hydrated alumina is commercial pseudoboehmite. The hydrated alumina is placed in sequence in the system after the calcium-exchanged zeolite X and before the selective sulfur adsorbent. Fifty percent of the components by volume is composed of the zeolite X and 25% is composed of each of the selective sulfur adsorbent and the hydrated alumina. A total of 10 ccs of the components is used. The operating conditions and the composition of the feed stream are the same as for Example 1. The percent S in the form of COS that was removed by the system is listed in FIG. 2. When the feed stream is passed through the reactor, breakthrough does not occur until 204 hours, as shown in FIG. 1. In addition, as shown in FIG. 2, a higher percentage of sulfur is removed from the feed stream by breakthrough utilizing the three c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com