Methods for treating renal cell carcinoma
a renal cell carcinoma and il2 technology, applied in the field of renal cell carcinoma treatment with il2, can solve the problems of severe side effects, neurological changes, fever and chills, etc., and achieve the effects of inhibiting tumor growth, reducing toxicity, and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Pharmaceutical Composition of IL-2 for Phase IV Human Clinical Trial
[0102] The IL-2 formulation used was manufactured by Chiron Corporation of Emeryville, Calif., under the tradename Proleukin®. The IL-2 in this formulation is a recombinantly produced, unglycosylated human IL-2 mutein, called aldesleukin, which differs from the native human IL-2 amino acid sequence in having the initial alanine residue eliminated and the cysteine residue at position 125 replaced by a serine residue (referred to as des-alanyl-1, serine-125 human interleukin-2). This IL-2 mutein is expressed in E. coli, and subsequently purified by diafiltration and cation exchange chromatography as described in U.S. Pat. No. 4,931,543, incorporated herein by reference in its entirety. The IL-2 formulation marketed as Proleukin® is supplied as a sterile, white to off-white preservative-free lyophilized powder in vials containing 1.3 mg of protein (22 MIU).
example 2
Selection Criteria for Patients with Metastatic Renal Cell Carcinoma for Treatment with IL-2 in Phase IV Human Clinical Trial
[0103] The following selection criteria were applied to patients with metastatic renal cell carcinoma:
[0104] Inclusion criteria: [0105] Patient has histologically documented renal cell carcinoma (clear cell, papillary, mixed or sarcomatoid tumors) with evidence of metastatic disease; [0106] Patient has measurable or evaluable neoplastic disease which is determined within four weeks of entering the study; [0107] Karnofsky Performance Status of patient is 60, corresponding to an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0-2; [0108] Patients must be greater than 18 years of age; [0109] Patients should have adequate renal function as evidenced by a serum creatinine level of less than 1.8 mg / dl; [0110] Hemoglobin 10 gm / dl; White blood cell 4,000 / ml; Platelet 100,000 / ml; [0111] Normal thyroid stimulating hormone (TSH) level; and [0112] P...
example 3
Phase IV Clinical Study of Low Dose IL-2 Administered to Humans
[0123] Clinical Study Design and Objectives
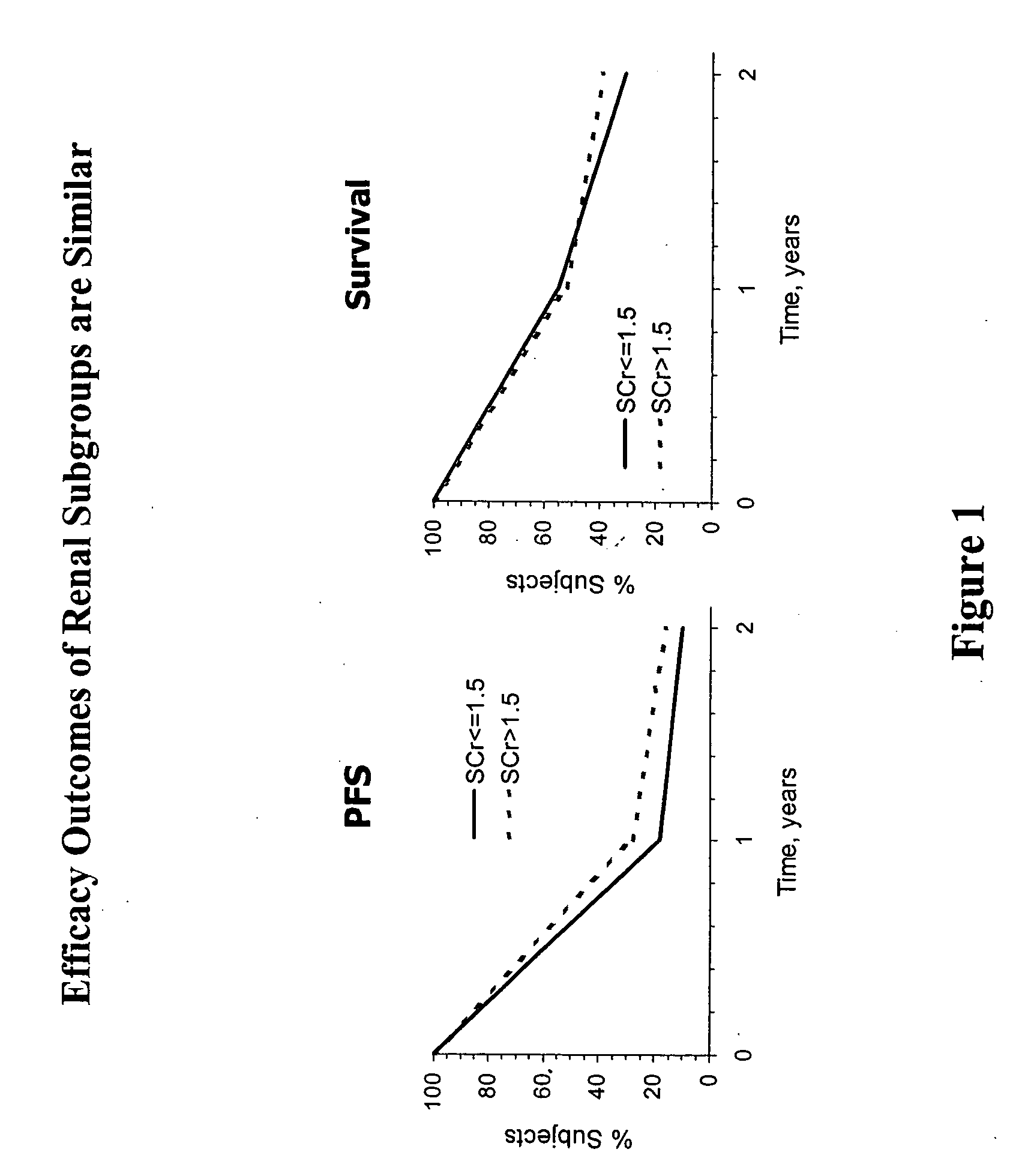
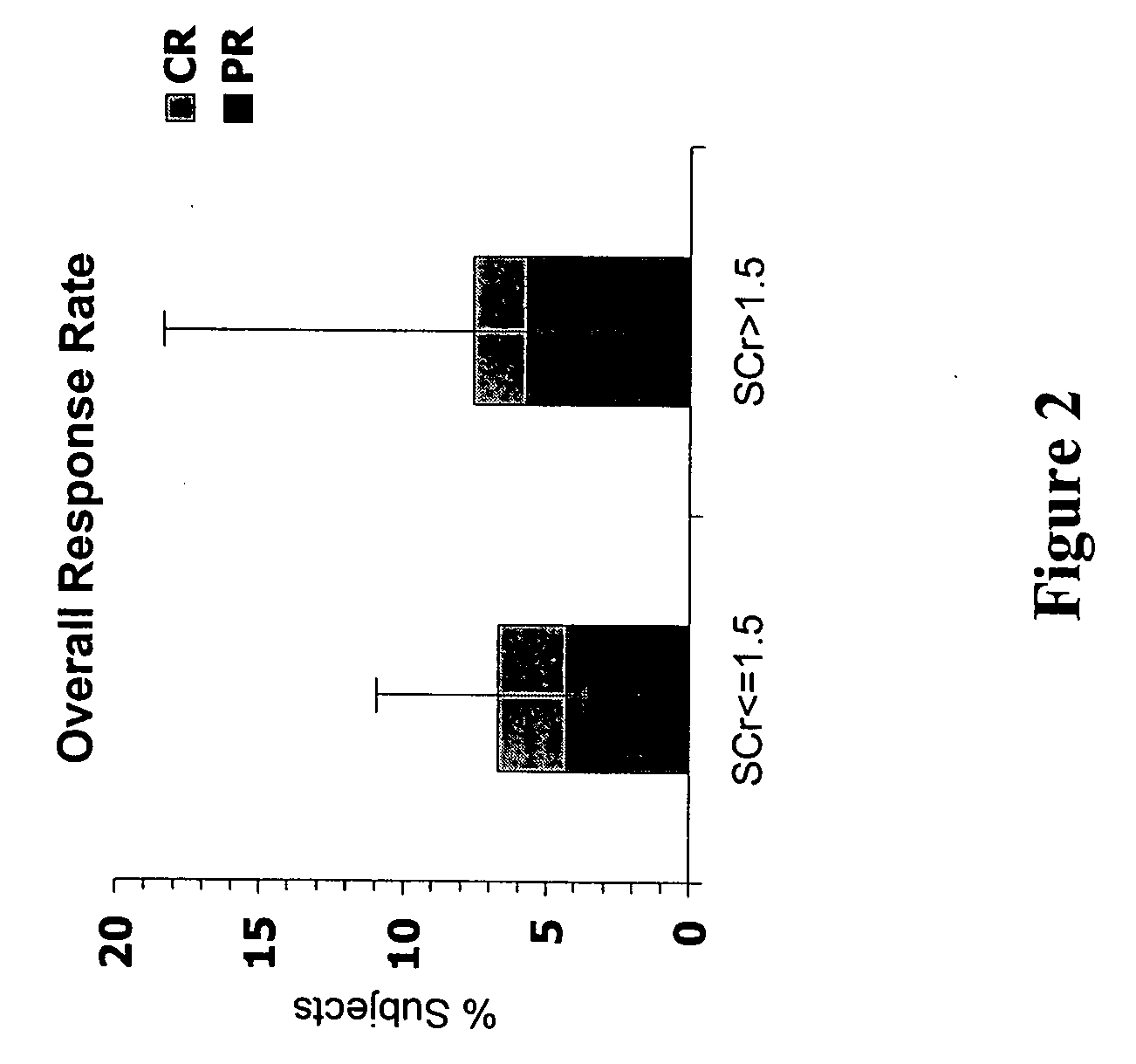
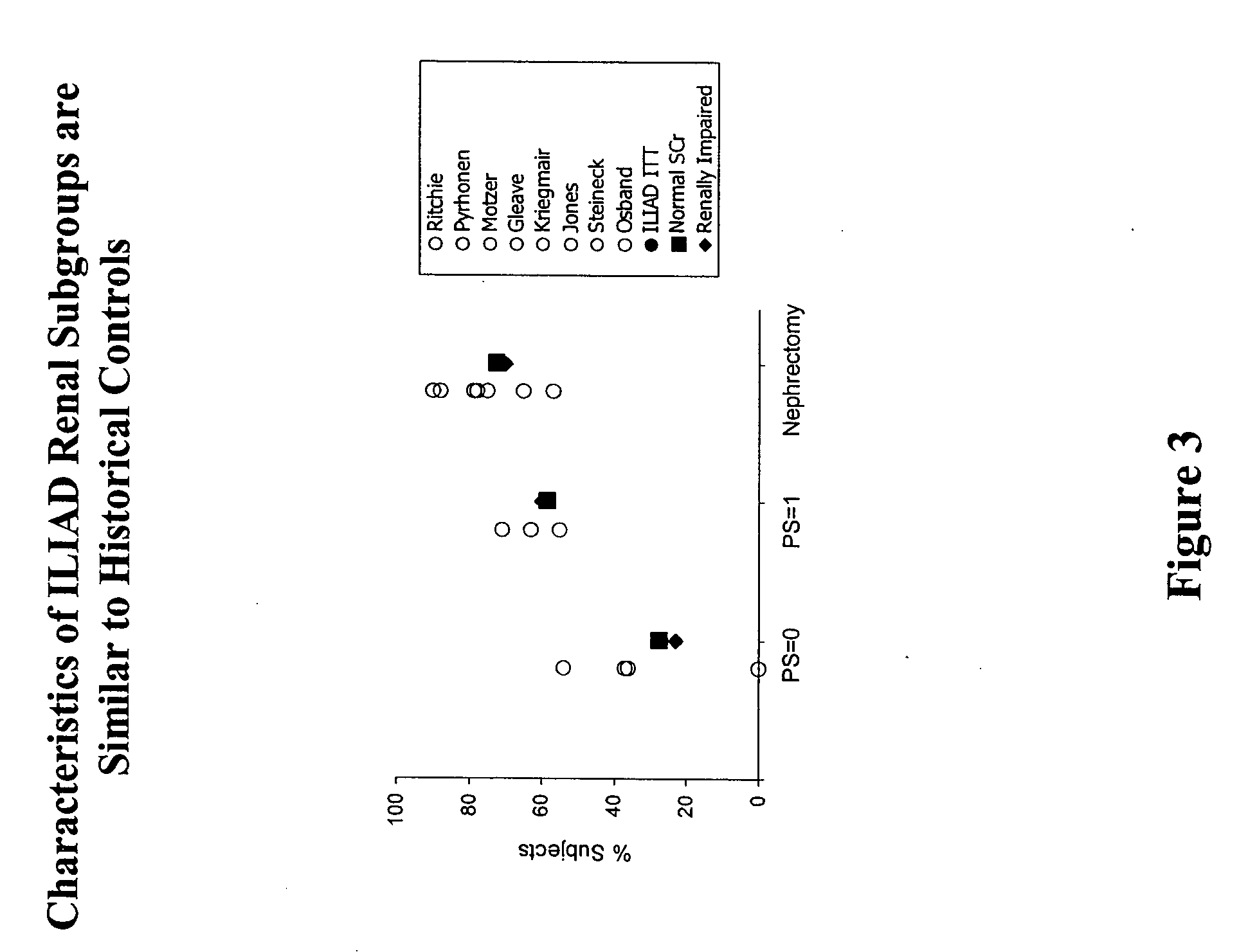
[0124] The Phase IV clinical study of interleukin-2 in an alternative dose (ILIAD) was designed as a prospective, multi-center, single-arm, open-label study to evaluate the efficacy and safety of low dose Proleukin® administered subcutaneously in patients with metastatic renal cell carcinoma. The primary endpoint was an objective response rate (ORR)≧16%. Secondary endpoints included complete response (CR) and partial response (PR) rates, duration of response, progression free survival (PF), overall survival (OS), and incidence of adverse events (AEs). Participants included clinical investigators from both community and academic settings, with a preponderance of community clinicians. The ILIAD study screened 270 patients, of which 267 were enrolled.
[0125] Pretreatment evaluations included a complete history, physical exam, complete blood count (CBC), serum chemistry panel, thy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com