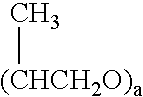Treatment of mucositis using N-acetylcysteine
a technology of nacetylcysteine and mucositis, which is applied in the field of therapeutic compositions, can solve the problems of pain, other cell types can be damaged as well, and significant disruption of cellular integrity in mucosal epithelium, so as to prevent or reduce the incidence, severity and/or duration of the diseas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
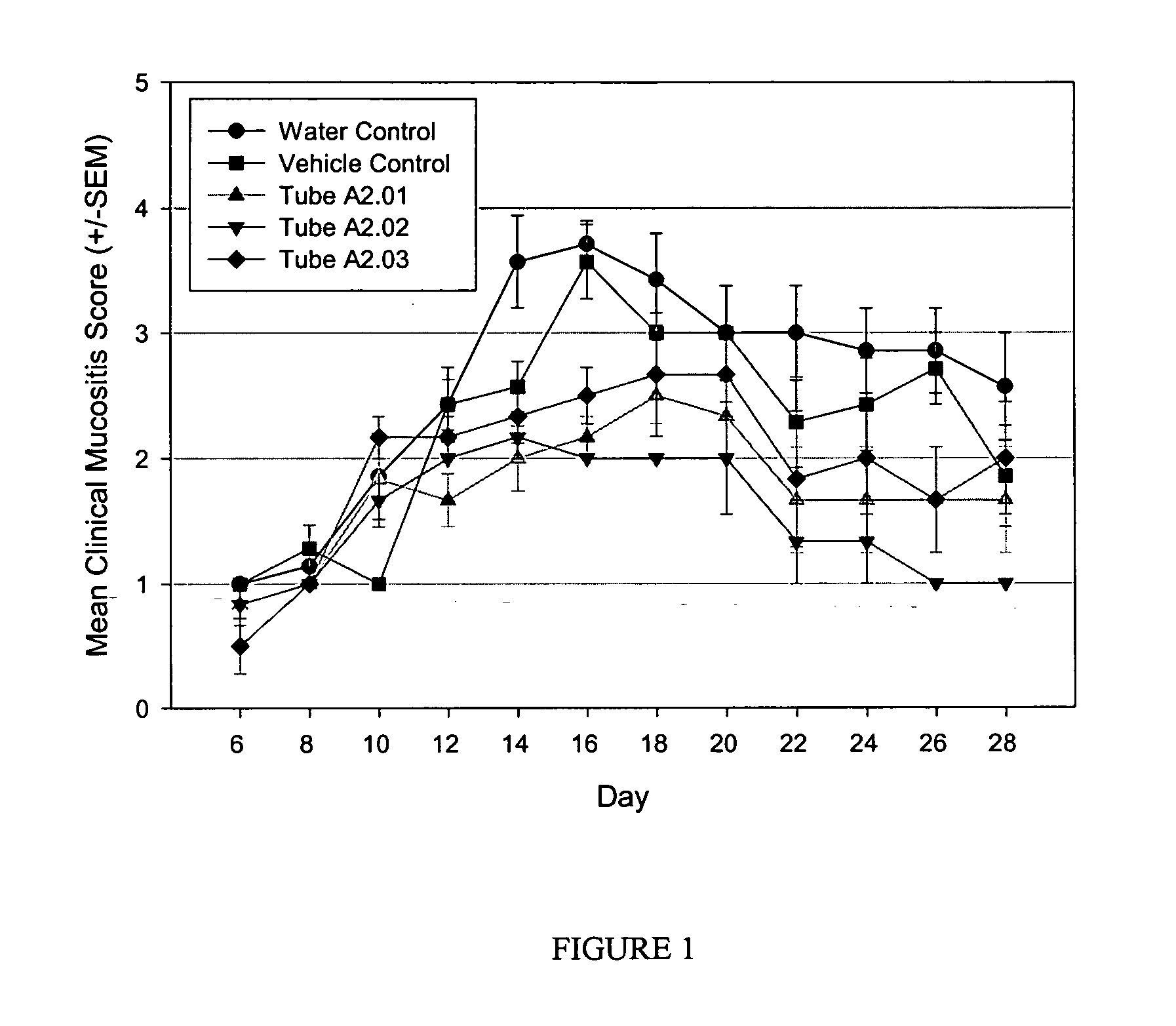
[0084] This example describes the formulation and use of the antioxidant, NAC, within a Pluronic® F127 delivery matrix in the absence and presence of chitosan as a penetration enhancer, for preventing or reducing the clinical outcome of oral mucositis in a hamster model of radiation-induced buccal mucositis.
[0085] Preparation of stock solutions: Pluronic® F127 (poloxamer 407; BASF Corporation, Washington, N.J.) was autoclaved and dissolved in sterile water for injection (Abbott Laboratories, North Chicago, Ill.) at 30% (w / w). Chitosan (medium molecular weight; Sigma-Aldrich, St. Louis, Mo.) was autoclaved and dissolved at 3% (w / w) in sterile filtered water for injection containing 1% (v / v) acetic acid (Fisher Scientific, Fair Lawn, N.J.). NaOH (Fisher Scientific) was prepared in sterile water for injection at 4 M and sterile filtered.
[0086] Preparation of antioxidant formulations: The antioxidant, N-acetyl-L-cysteine (NAC; Sigma-Aldrich), was formulated in the various delivery mat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight percent | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com