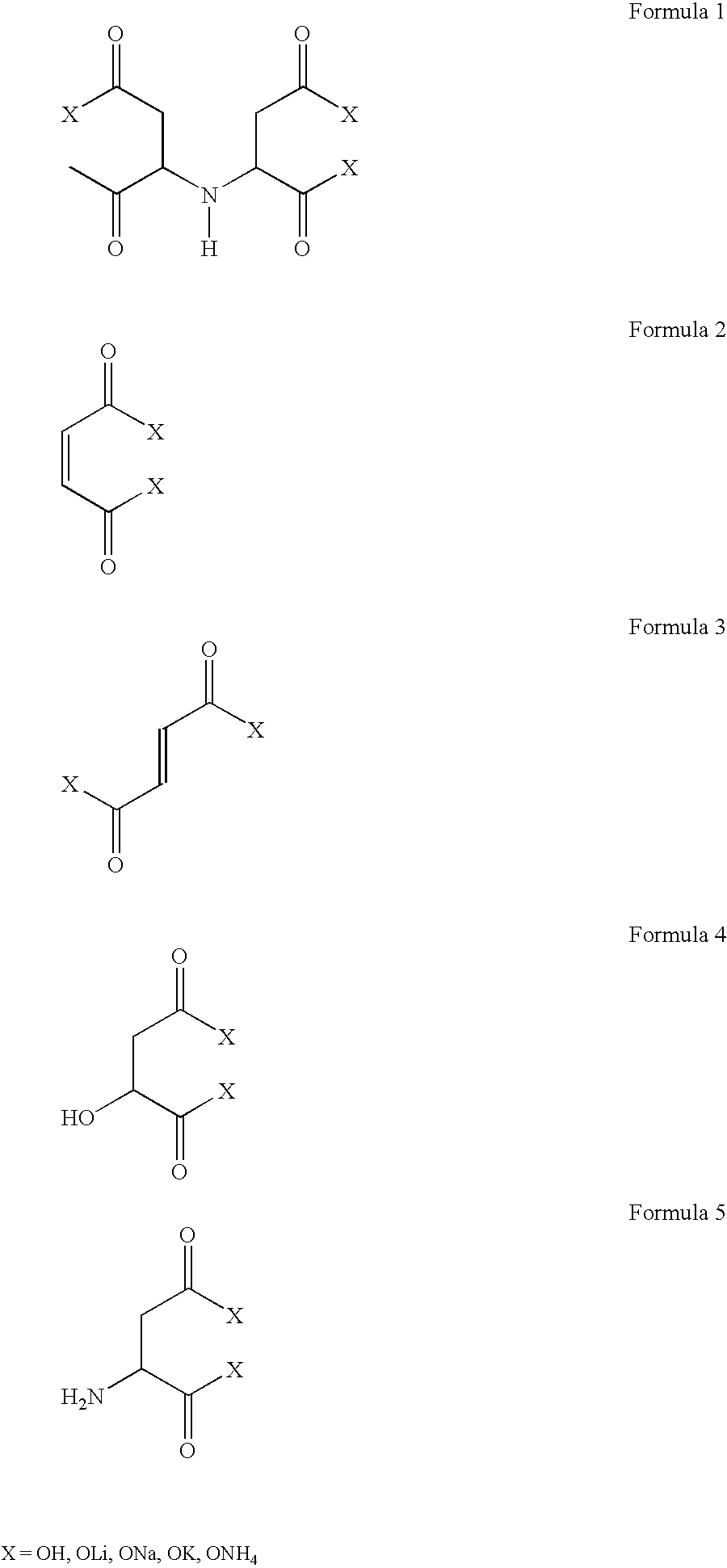
Preparation and use of iminodisuccinic acid ammonium metal salts
a technology iminodisuccinic acid, which is applied in the field of process for the preparation of ammonium metal salt of iminodisuccinic acid ammonium salt, can solve the problems of only partially or not at all biodegradabl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0060] MA:NH3:H2O molar ratio=2:4:9, 90° C., 30 h
[0061] 1 782 g=99 mol of water are introduced and heated to 80° C. 2 157.3 g=22 mol of maleic anhydride are added at 80-100° C. with stirring and cooling. After the maleic anhydride has gone into solution, the mixture is stirred for a further 0.5 h approximately. Subsequently, 748 g=44 mol of ammonia are introduced at >90° C. After the introduction of ammonia has ended, the reaction mixture is stirred at 90° C. for more than 30 h, diluted with 1 312.7 g=72.93 mol of water and cooled to ambient temperature. After a clarifying filtration, which is optionally carried out, 6 000 g of product solution are obtained with the following yields: 80.0% of theory iminodisuccinic acid ammonium salt, 14.6% of theory aspartic acid ammonium salt, 2.5% of theory fumaric acid ammonium salt, 0.6% of theory malic acid ammonium salt and 0.4% of theory maleic acid ammonium salt. The solids content (=Σ ammonium salts) is 55 weight %. The clear light-yellow...
example 2
[0062] MA:NH3:H2O molar ratio=2:4:9, 90° C., 24 h
[0063] Implementation and amounts of this example correspond to those of Example 1. The reaction time is 24 h. The following yields are obtained: 77.9% of theory iminodisuccinic acid ammonium salt, 12.8% of theory aspartic acid ammonium salt, 3.0% of theory fumaric acid ammonium salt, 1.0% of theory malic acid ammonium salt and 3.4% of theory maleic acid ammonium salt.
example 3
[0064] MA:NH3:H2O molar ratio=2:4:9, 90° C., 36 h
[0065] Implementation and amounts of this example correspond to those of Example 1. The reaction time is 36 h. The following yields are obtained: 80.1% of theory iminodisuccinic acid ammonium salt, 15.0% of theory aspartic acid ammonium salt, 3.3% of theory fumaric acid ammonium salt, 1.1% of theory malic acid ammonium salt and 0.6% of theory maleic acid ammonium salt. The solids content (=Σ ammonium salts) is 55 weight %. The clear light-yellow solution has a density of 1.243 kg / litre and a pH of 7.03.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com