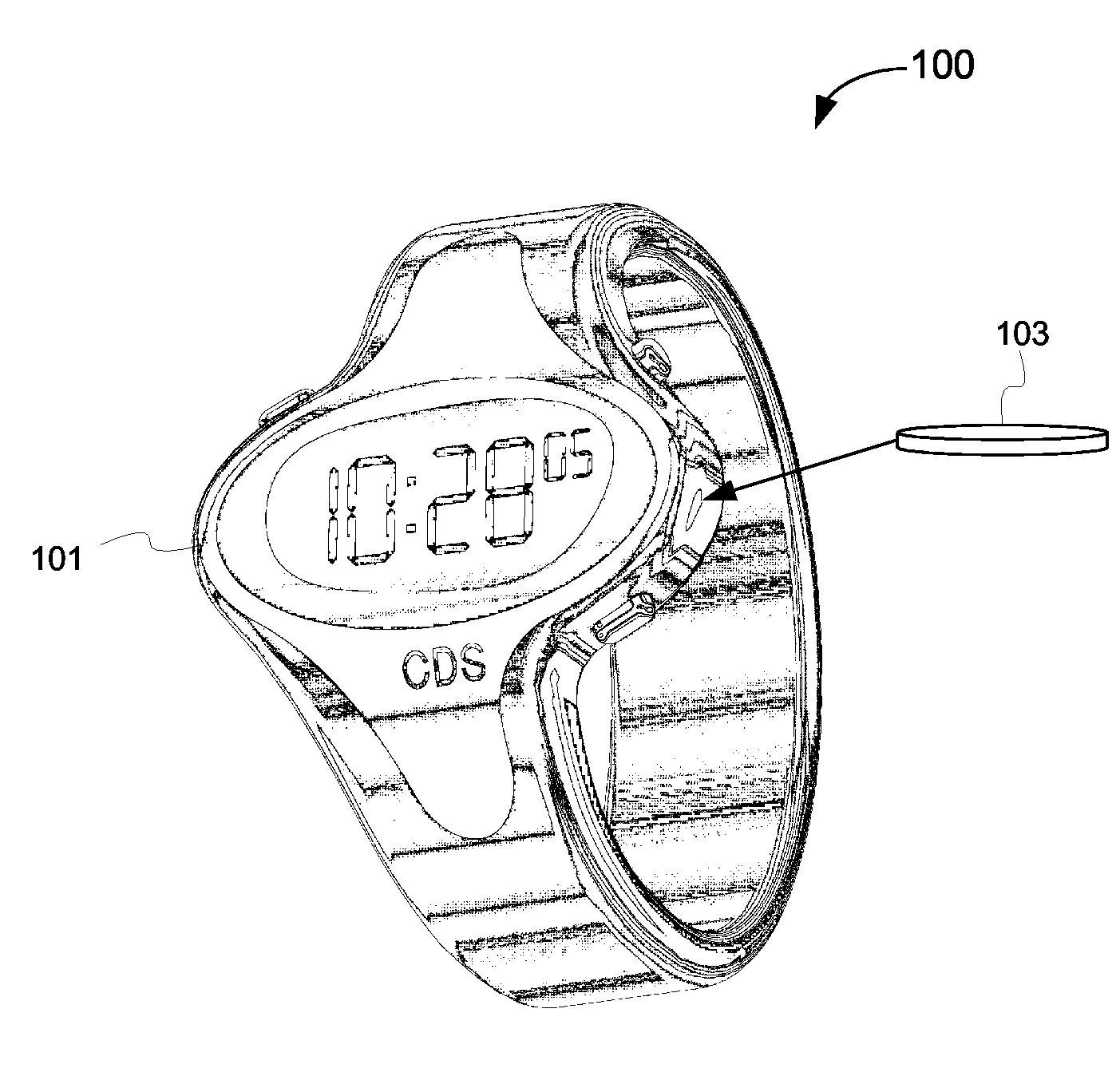
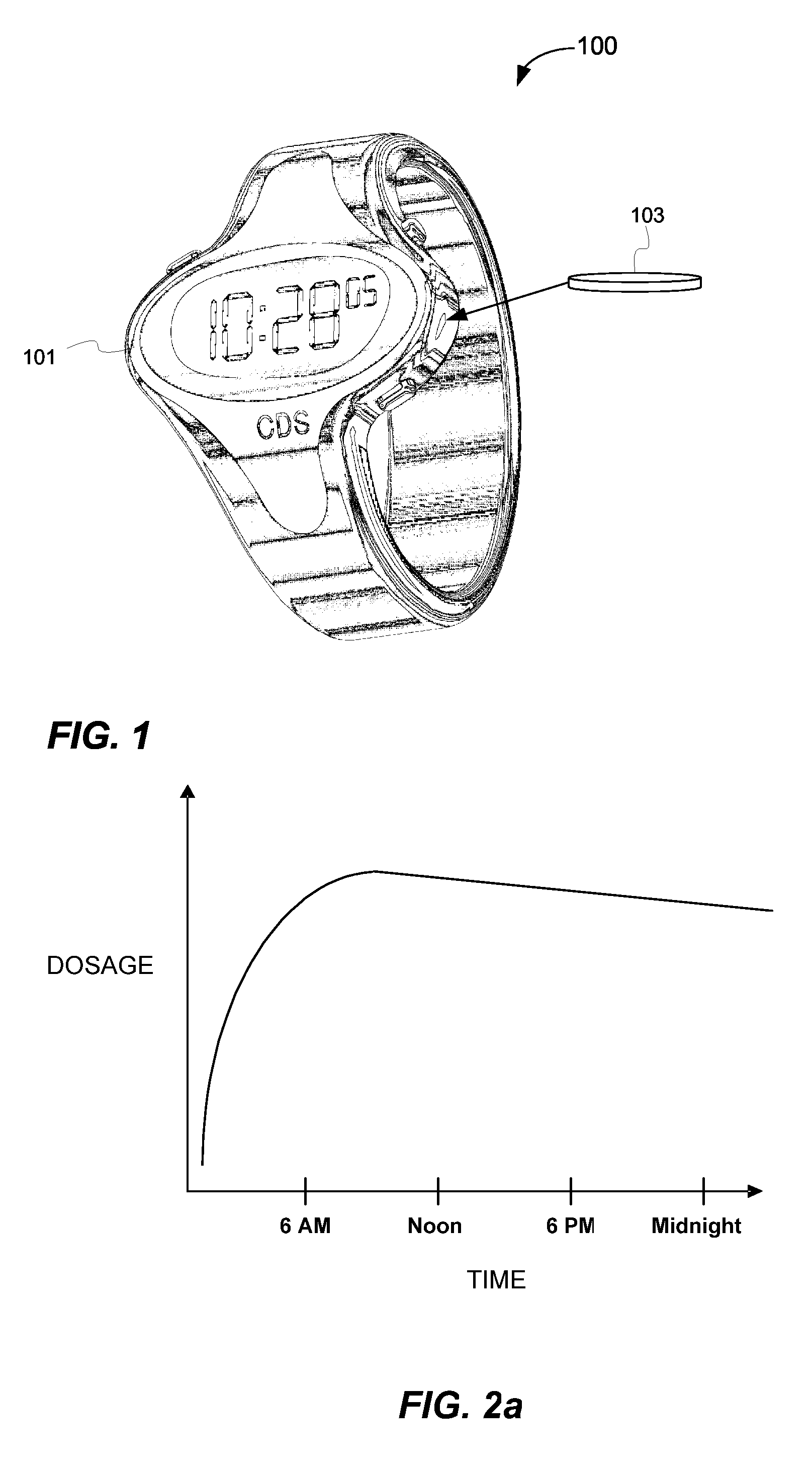
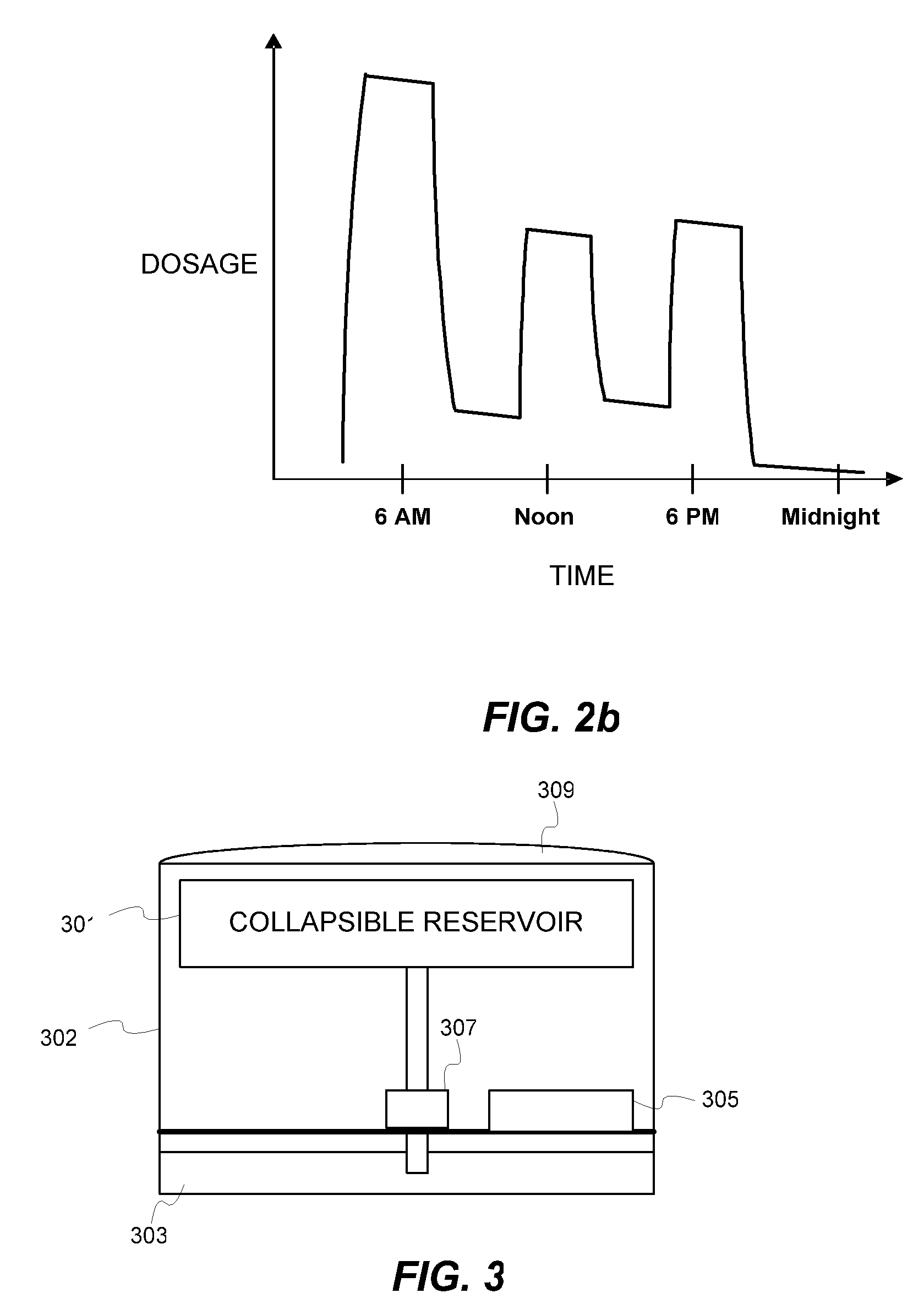
Biosynchronous transdermal drug delivery
a biosynchronous, drug technology, applied in the direction of infusion needles, nitro compound active ingredients, therapy, etc., can solve the problems of undetectable invasiveness of administration, difficult control of sustained release systems, and variability, so as to minimize negative side effects and less drugs, the effect of increasing the effect of the dosing regimen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example substances
[0041] Example substances include caffeine and a variety of over-the-counter and prescription stimulants (for treating fatigue, sleep disorders, attention deficit disorders and a variety of other conditions), nicotine (for smoking cessation), nitroglycerin (for treating heart attack and strokes), fentanyl (for treating chronic pain), albutamol (for treating asthma), and selegiline (for treating depression, attention deficit disorder or Parkinson's disease). We have carefully identified these specific drugs and diseases because they have the following attributes: (i) Chrono-Pharmacology is critical to optimized dosing but is not being implemented because no automated transdermal system exists, and (ii) these drugs can be transdermally absorbed passively (i.e., without the need for ultrasound or electrical stimulation or other permeation enhancers). Exemplary chrono-pharmacological systems that can make use of the present invention are summarized in Table 1
DISEASES / CONDITIONCHRONOPHAR...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| bioactive | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com