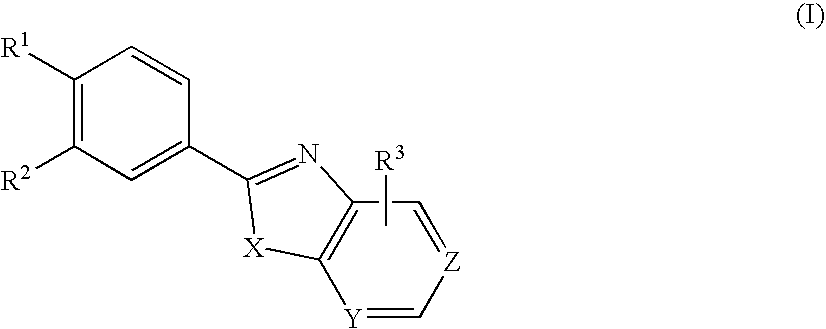
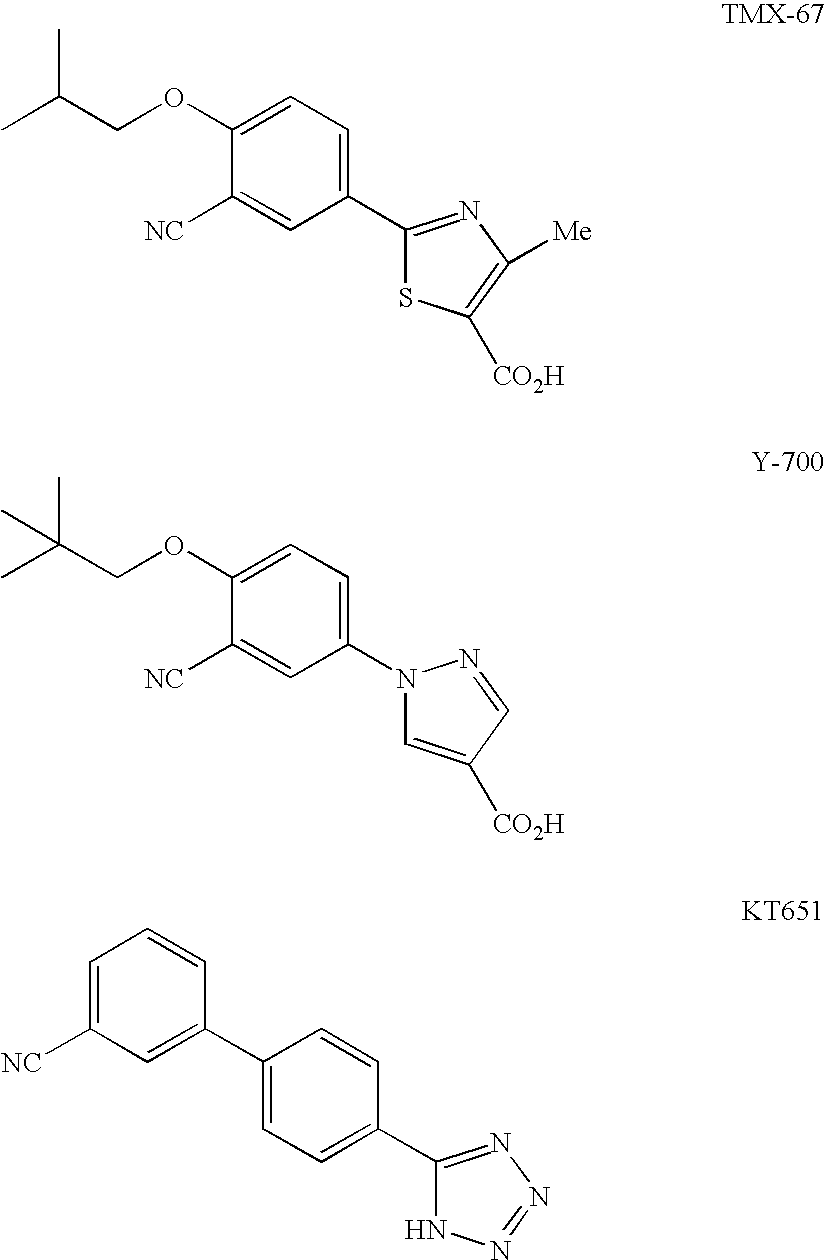
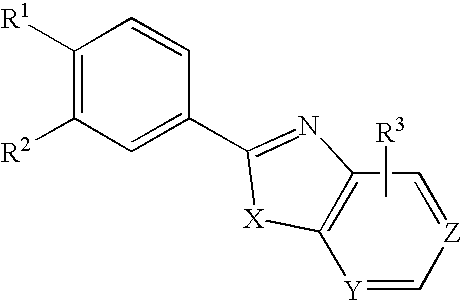
Xanthine Oxidase Inhibitor
a technology of xanthine oxidase and xanthine, which is applied in the direction of drug composition, extracellular fluid disorder, metabolic disorder, etc., can solve the problems of increased chance of side effects, lesion, and hepatopathy of allopurinol,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
8-(4-Isobutoxy-3-nitrophenyl)-6-chloropurine(A) and 6-amino-8-(4-isobutoxy-3-nitrophenyl)15 oxazolo[4,5-d]pyrimidine(B)
(1) Methyl 4-hydroxy-3-nitrobenzoate
[0071] 4-Hydroxy-3-nitrobenzoic acid (10.0 g, 54.6 mmol) was suspended in methanol (60 mL). After addition of conc. sulfuric acid (0.1 mL), the suspension was heated overnight under reflux. The methanol was distilled off under reduced pressure. The residue was dissolved in ethyl acetate (40 mL), washed successively with aqueous sodium hydrogen carbonate (20 mL×2) and saturated aqueous brine (20 mL), and dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure, to give 6.27 g (yield 58%) of the desired compound in the form of a pale brown crystalline product.
[0072] M. p. 72-73° C. 1H NMR (CDCl3, 400 MHz) δ: 3.95 (3H, s), 7.22 (1H, d, J=9 Hz), 8.24 (1H, dd, J=2 Hz, 9 Hz), 8.83 (1H, d, J=2 Hz), 10.89 (1H, s).
(2) Methyl 4-isobutyloxy-3-nitrobenzoate
[0073] Isobutyl bromide (10.5 mL, 95.8 mmol) was ...
example 2
8-(4-Isobutoxy-3-nitrophenyl)-6-hydroxypurine
[0085] A suspension of 8-(4-isobutoxy-3-nitrophenyl)-6-chloropurine (30 mg, 0.086 mmol) in aqueous 2M hydrochloric acid (4.5 mL) was heated to 120° C. for 3 hours under stirring. The suspension was then cooled to room temperature. The precipitated solid product was collected by filtration, washed with water, and dried in air. The dried product was heated to 100° C. after addition of aqueous 2M hydrochloric acid (27 mL). The insolubles were removed while the aqueous portion was still hot. The filtrate was stirred overnight. The precipitated solid product was collected by filtration, washed with water, and dried in air, to give 12 mg (yield 43%) of the desired compound in the form of a yellow powdery product.
[0086] hu 1H NMR (DMSO-d6, 400 MHz) δ: 1.00 (6H, d, J=7 Hz), 2.0-2.2 (1H, m), 4.03 (2H, d, J=6 Hz), 7.55 (1H, d, J=9 Hz), 8.02 (1H, s), 8.39 (1H, dd, J=2 Hz, 9 Hz), 8.65 (1H, d, J=2 Hz), 12.28 (1H, s).
[0087] FAB-MS (m / e): 330 (M+1). ...
example 3
8-(4-Isobutoxy-3-nitrophenyl)-1H-benzimidazole
[0088] 1,2-Phenylenediamine (1.09 g, 10.1 mmol) was dissolved in THF (20 mL). The solution was cooled with ice water, and to the cooled solution was dropwise added to a solution of 4-isobutoxy-3-nitrobenzoyl chloride (1.00 mmol) in THF (3 mL) for more than 30 min. The mixture was then stirred for 3 hours under cooling with ice-water. The solvent was distilled off under reduced pressure. The residue was suspended in water (20 mL) and stirred for 30 min., at room temperature. The obtained crystalline product was collected by filtration, washed with water (5 mL×3), and dried at room temperature under reduced pressure, to give 291 mg (yield 88%) of the desired amide product in the form of a pale yellow crystalline product.
[0089] The amide product (165 mg, 0.50 mmol) and phosphoryl chloride (3.0 mL) were together heated under reflux for 3 hours, and then allowed to stand and cooled to room temperature. The reaction mixture was poured into i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com