Production method of polymers by using living anionic polymerization method
a technology of anionic polymerization and production method, which is applied in the field of producing polymers by using a living anionic polymerization method, can solve the problems of increasing the relative effect of polymerization inhibiting substances, difficult control of anionic polymerization under the presence of these materials, and prone to deactivation of growing terminal anion, etc., and achieves high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
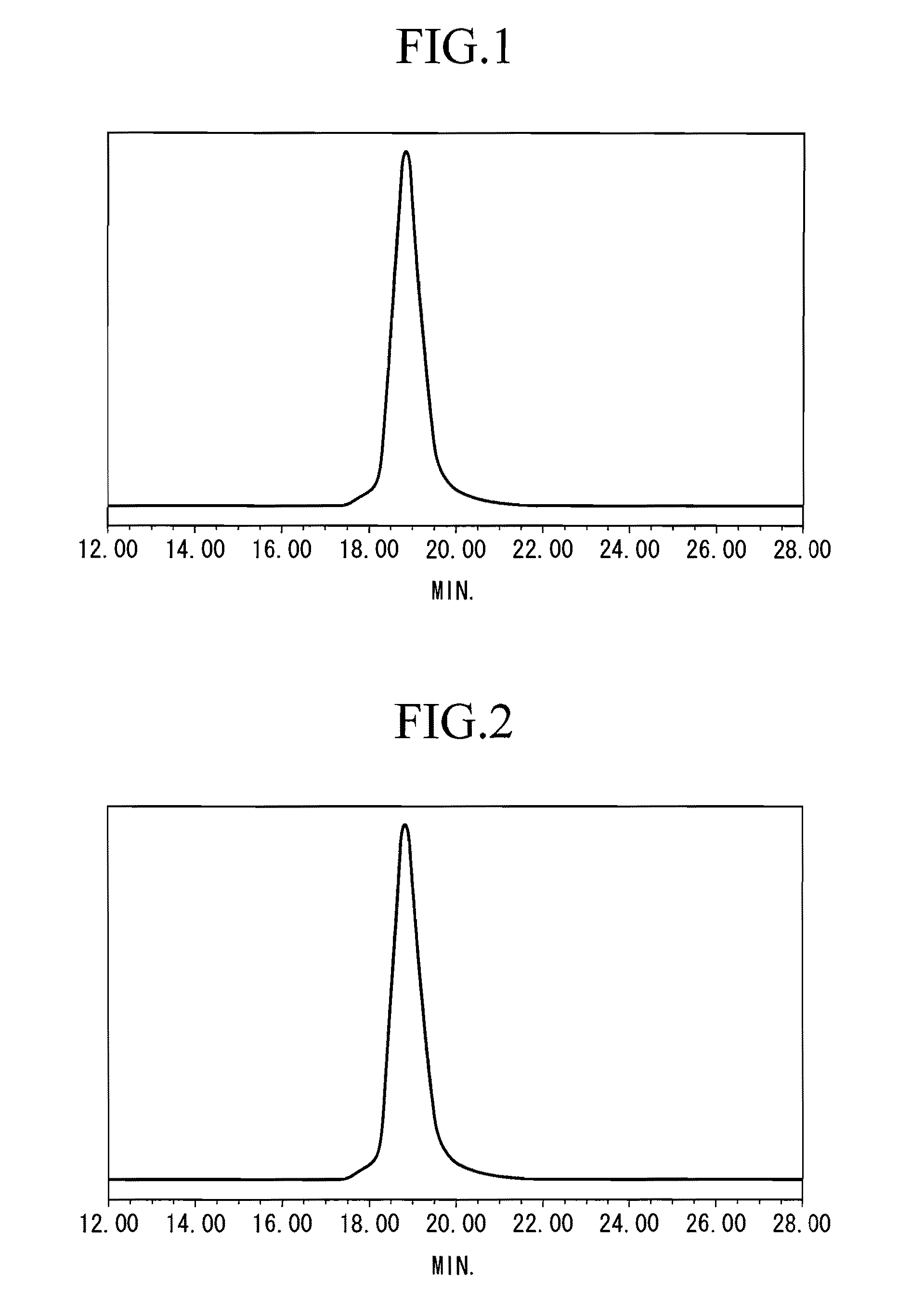
[0096] After adding 2.56 g (3.67 mmol) of a dibutyl magnesium solution (DBuMg) to 570 g of THF under a nitrogen atmosphere at −40° C., 29.8 g (286.1 mmol) of styrene was added thereto and the resulting mixture was stirred for 10 minutes. 1.15 g (2.76 mmol) of n-BuLi solution was added to the resulting solution and the entire mixture was stirred at −40° C. for 30 minutes. 11.2 g (63.7 mmol) of p-t-butoxystyrene (PTBST) was then added to this reaction solution and further stirred at −40° C. for 90 minutes. After stopping the reaction by adding methanol to the obtained reaction mixture, the resulting mixture was subjected to a reprecipitation process using a methanol solvent to obtain a reprecipitate, then the reprecipitate was filtered to obtain the retained matter, and the retained matter was air-dried to obtain styrene-PTBST copolymer A (yield 99%). The Mw of the copolymer A was 219,500 and the ratio Mw / Mn was 1.15.
[0097] The GPC curve of copolymer A is shown in FIG. 1. The horizon...
example 2
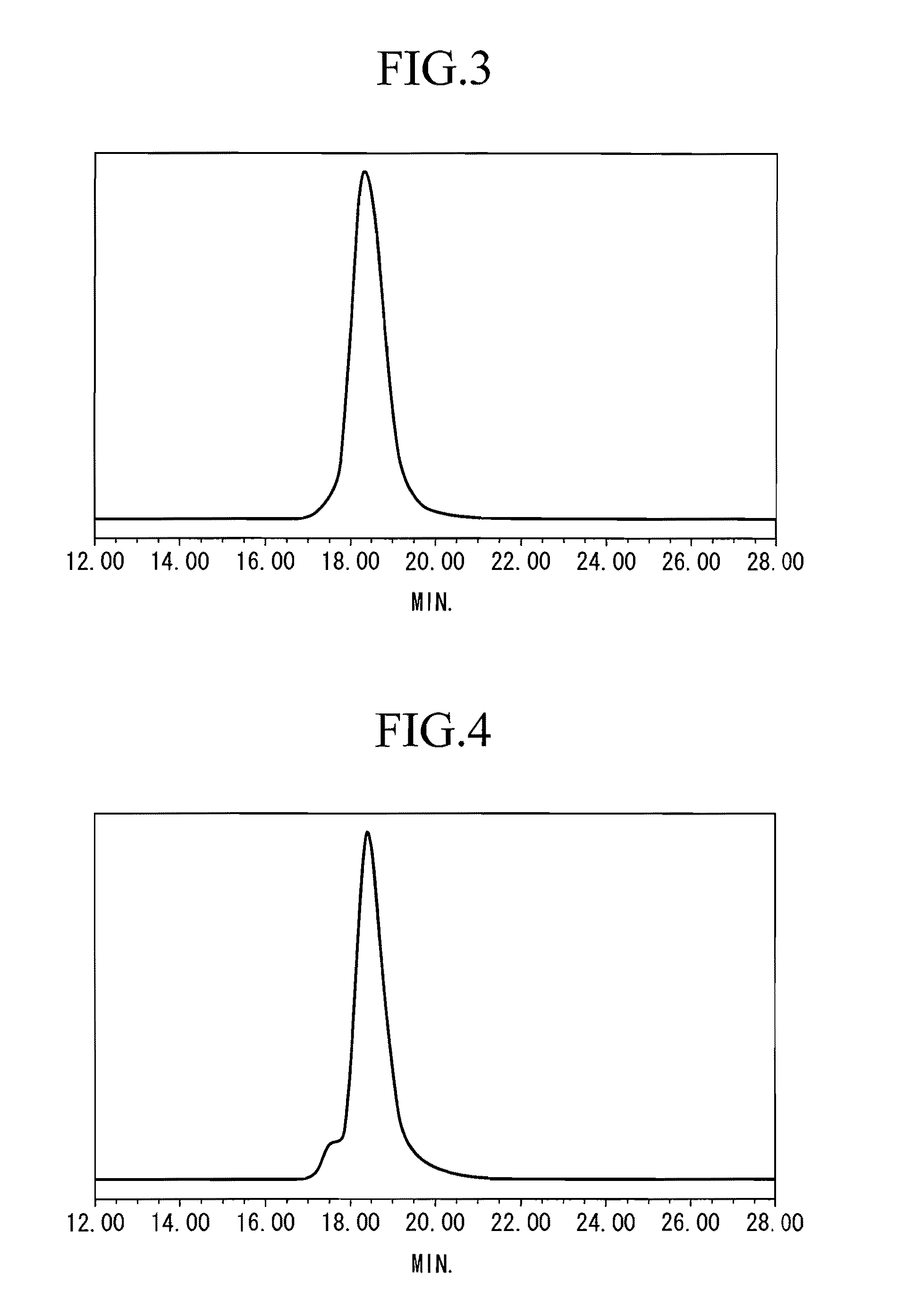
[0100] After adding 2.46 g (3.46 mmol) of a dibutyl magnesium solution (DBuMg) to 570 g of THF under a nitrogen atmosphere at −40° C., 32.7 g (314.0 mmol) of styrene was added thereto and the resulting solution was stirred for 15 minutes. 1.01 g (2.42 mmol) of n-BuLi solution was added to this solution and the resultant solution was stirred at −40° C. for 30 minutes. A mixed solution of 0.40 g (0.56 mmol) of DBuMg and 12.7 g (72.1 mmol) of p-t-butoxystyrene (PTBST) was then added to this reaction solution and the resulting solution was further stirred at −40° C. for 90 minutes. After stopping the reaction by adding methanol to the obtained reaction mixture, the resulting mixture was subjected to a reprecipitation process using a methanol solvent to obtain a reprecipitate, then the reprecipitate was filtered to obtain the retained matter, and the retained matter was air-dried to obtain styrene-PTBST copolymer C (yield 99%). The Mw of the copolymer C was 335,550 and the ratio Mw / Mn wa...
example 3
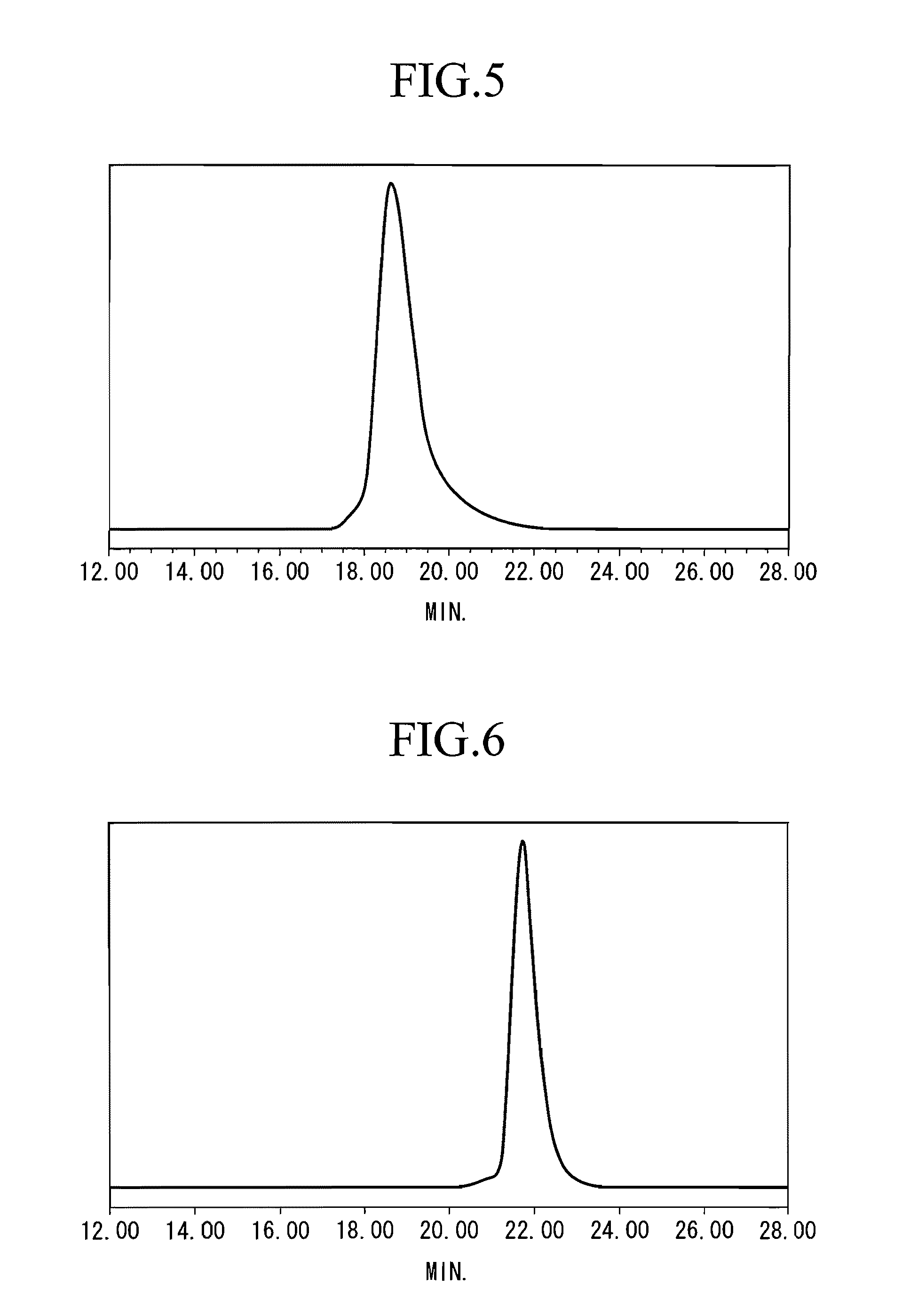
[0102] After adding 3.80 g (5.35 mmol) of a dibutyl magnesium solution (DBuMg) to 570 g of THF under a nitrogen atmosphere at −40° C., 30.8 g (297.6 mmol) of styrene was added thereto and the resulting solution was stirred for 10 minutes. 1.02 g (2.45 mmol) of n-BuLi solution was added to the solution and the resultant solution was stirred at −40° C. for 30 minutes. 6.43 g (33.4 mmol) of 4-(2-ethoxy-ethoxystyrene) (PEES) was then added to this reaction solution and the resulting solution was further stirred at −40° C. for 3 hours. After stopping the reaction by adding methanol to the obtained reaction mixture, the resulting mixture was subjected to a reprecipitation process using a methanol solvent to obtain a reprecipitate, then the reprecipitate was filtered to obtain the retained matter, and the retained matter was air-dried to obtain St-PEES copolymer D (yield 99%). The Mw of the copolymer D was 282,000 and the ratio Mw / Mn was 1.19.
[0103] The GPC curve of this copolymer D is sh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com