Method for Production of Metal by Molten-Salt Electrolysis and Method for Production of Titanium Metal
a technology of molten salt and metal, which is applied in the field of metal recovery, can solve the problems of reducing the yield of metal, limiting the efficiency of metal recovery, and difficult to recover metal such as calcium metal efficiently by conventional methods, and achieves the effect of reducing the solubility of metal in molten sal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
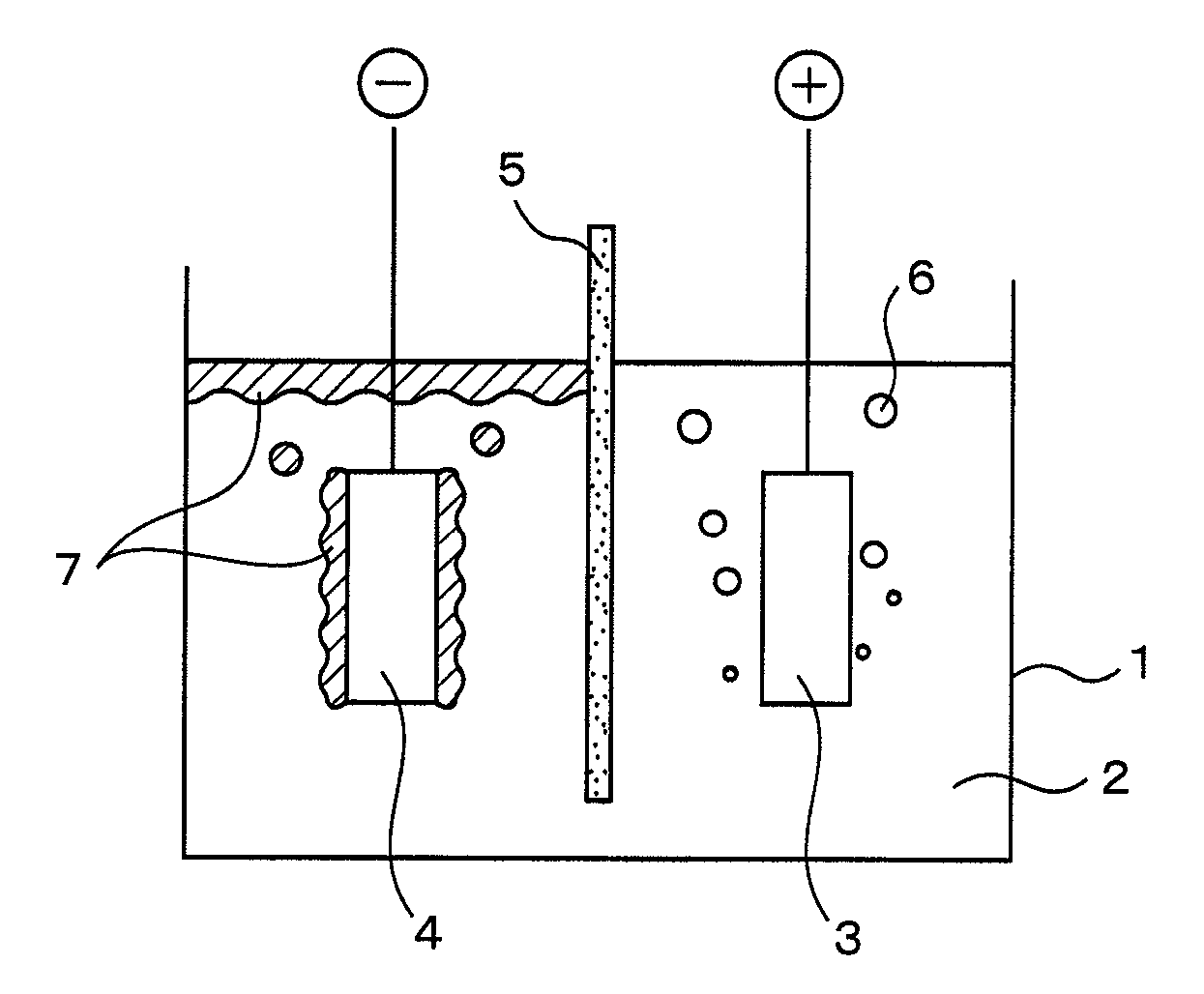
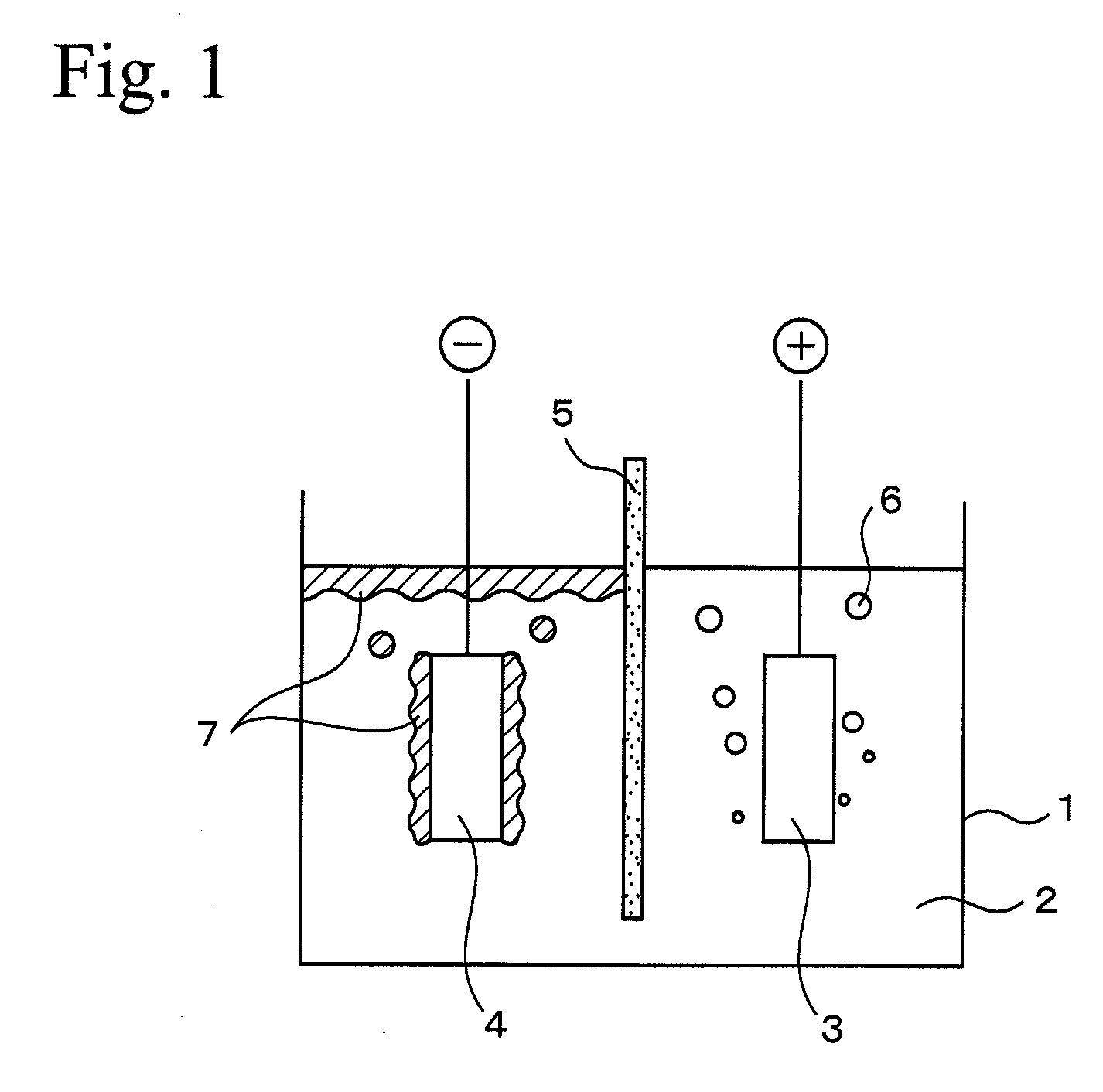
[0042] Using the electrolysis vessel shown in FIG. 1, while maintaining the temperature of the electrolysis bath consisting of calcium chloride at 75 mol % and potassium chloride at 25 mol % at 650° C., and applying a voltage of 4.5 V between an anode 3 made of carbon and the cathode 4 made of carbon steel, the electrolysis of the molten salt of calcium chloride is started. Accompanied by the electrolysis of the molten salt, calcium metal is deposited on the cathode in a solid state. After depositing a predetermined amount of calcium metal on the cathode in a solid state, electric power supply to the positive and cathodes is stopped. After that, the cathode, having deposited calcium metal on its surface, is transferred to a recovery vessel which is heated to a temperature not less than the melting point of calcium metal, and the calcium metal deposited on the surface of the cathode is melted so that it can be recovered. The ratio of the amount of calcium metal actually recovered to ...
example 2
[0043] Using the electrolysis vessel shown in FIG. 1, while maintaining the temperature of the electrolysis bath consisting of calcium chloride at 85 mol % and potassium chloride at 15 mol % at 730° C., and applying a voltage of 5.0 V between an anode 3 made of carbon and the cathode 4 madc of low-carbon steel, the electrolysis of the molten salt of calcium chloride was started. Accompanied by the electrolysis of the molten salt, calcium metal in a solid state floated up to the bath surface around the cathode. The electrolysis bath and calcium metal were drawn off and recovered from the bath surface around the cathode. The recovered calcium content in the electrolysis bath was measured to be 50%. The amount of calcium metal generated was measured from the recovered amount and the concentration, and a ratio was calculated with a theoretical generated amount calculated from the time of electric power supply. As a result, it was confirmed that not less than 75% of calcium metal was rec...
example 3
[0044] Using the electrolysis vessel shown in FIG. 1, while maintaining the temperature of the electrolysis bath consisting of calcium chloride at 85 mol % and potassium chloride at 15 mol % at 950° C., and applying a voltage of 5.0 V between an anode 3 made of carbon and a cathode 4 made of low-carbon steel, the electrolysis of the molten salt of calcium chloride was started. Accompanied by molten-salt electrolysis, calcium metal in a melted state floated up to the bath surface around the cathode. The electrolysis bath and melted calcium metal were drawn off and recovered from the bath surface around the cathode. Melted calcium was recovered and the concentration of calcium in the electrolysis bath which was recovered was measured and was 30%. The amount of calcium metal generated was measured from the recovered amount and the concentration, and a ratio with a theoretical generated amount calculated from the time of electric power supply was calculated. As a result, it was confirme...
PUM
| Property | Measurement | Unit |
|---|---|---|
| eutectic temperature | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| eutectic temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com