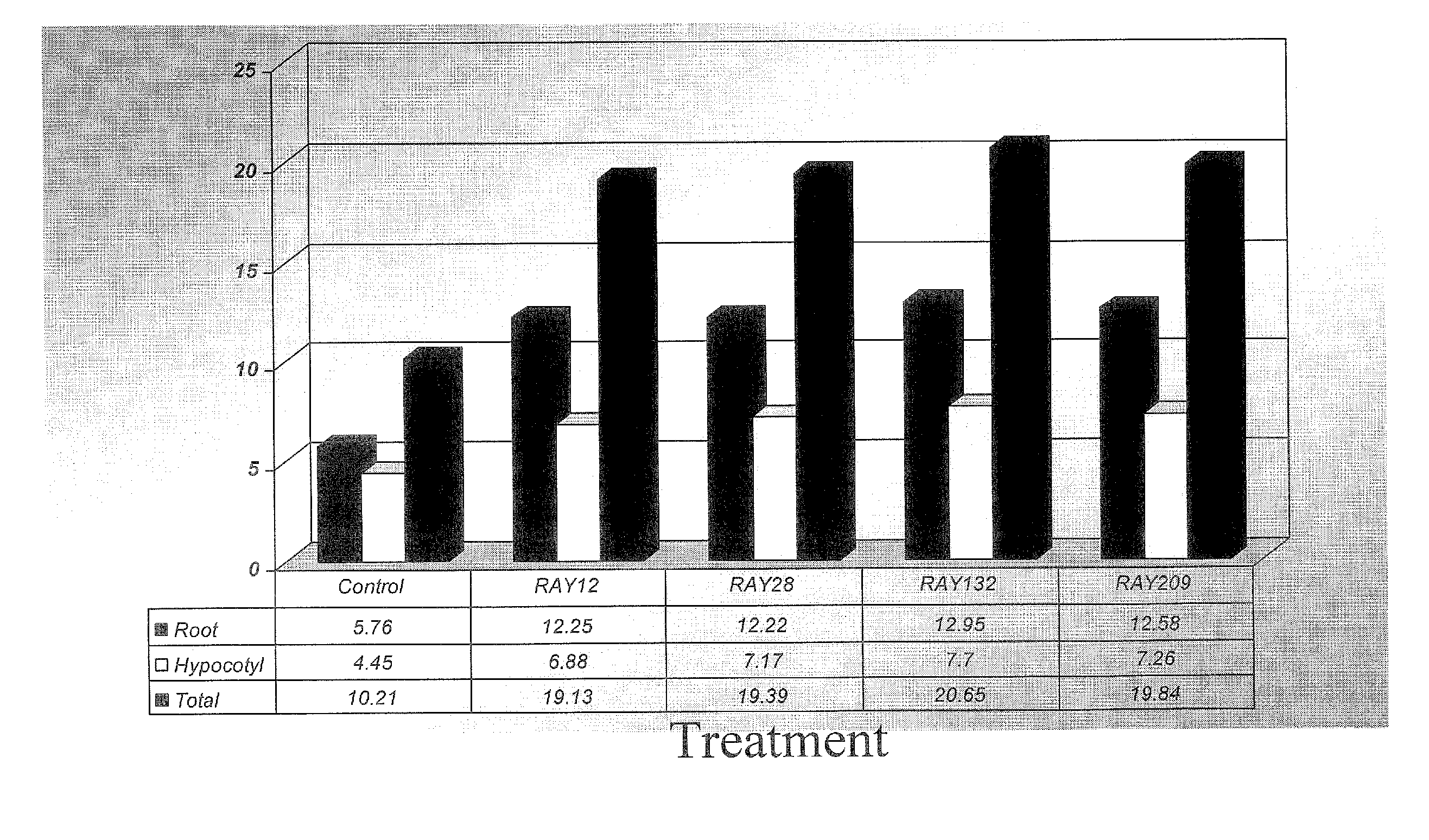Sulfur-oxidizing plant growth promoting rhizobacteria for enhanced canola performance
a technology of sulfur-oxidizing plant growth and promoting rhizobacteria, which is applied in the field of seed treatment, can solve the problems of difficult demonstration of the unfavorable effects of plant productivity, serious impact on the yield of canola in soils with low s-supplying capacity, and little work on these aspects. , to achieve the effect of reducing the use of fungicides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Sulfur-Oxidizing Rhizobacteria Isolation
[0054] Presumptive S-oxidizing rhizobacteria were isolated by plating serial dilution of the canola rhizosphere soil and rhizoplane (Grayston and Germida, 1991). The TSA (trypticase soy agar, 1 / 10 strength) media was used as the laboratory basal media. Laboratory modified two enrichment sulfur media were used for presumptive S-oxidizing bacterial isolation purpose. The thiosulphate and flowable elemental sulfur (FS) were used as suitable S source in the two different media. Flowable sulfur (Stoller Enterprises, Inc., Houston) is a brownish yellow colored creamy liquid with impurities and contains approximately 52% of elemental S. The FS was cleaned with distilled water. This FS was added to the media to provide the final concentration of 0.2% S in the solid media (i.e. TSA) and 1% S in the liquid media (i.e. trypticase soy broth, TSB). In the media bromothymol blue indicator was also used to record the change in media pH. Media plates were ex...
example 2
Quantitative Test of Sulfur Oxidation
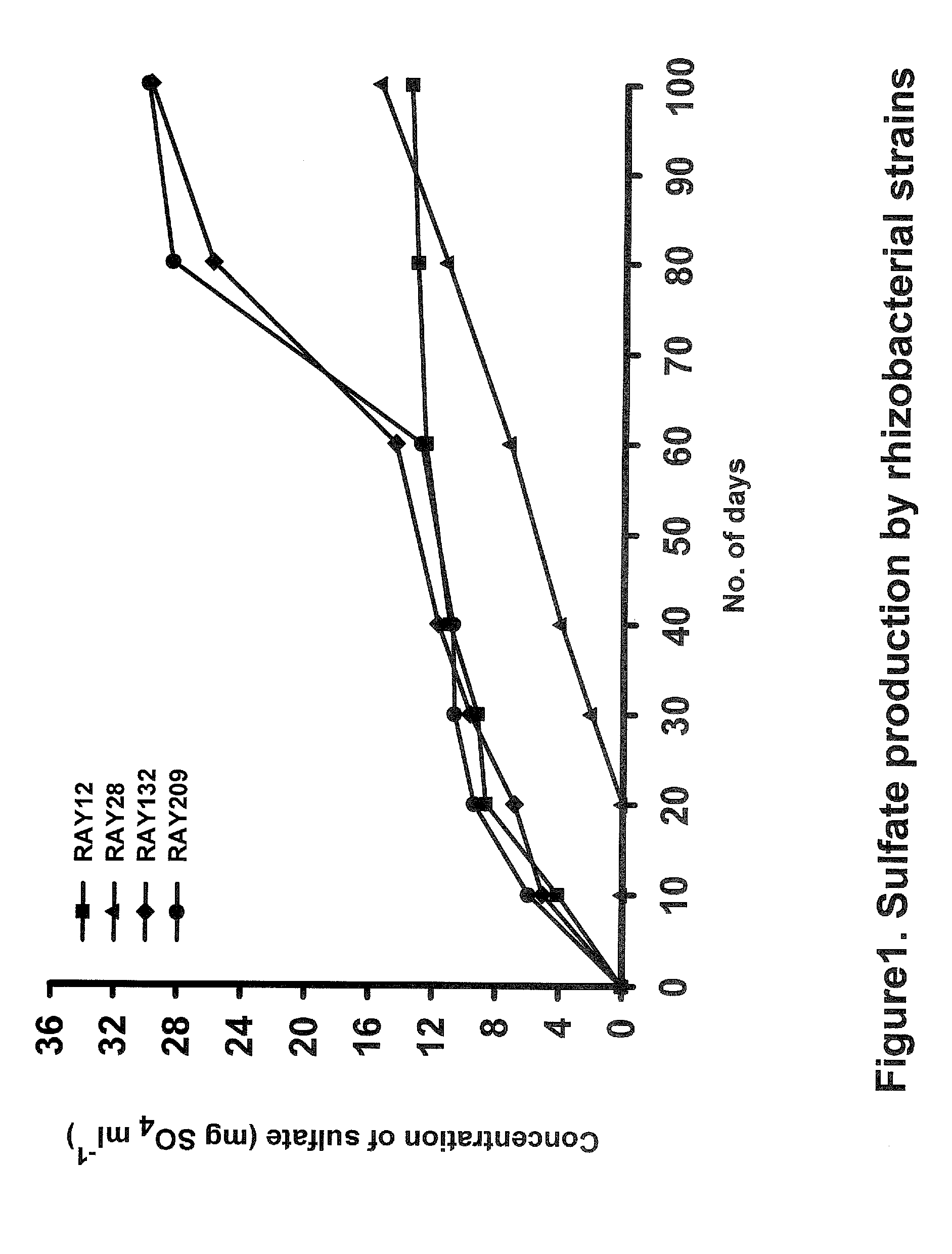
[0057] For the determination of quantitative bacterial S-oxidation an incubation study was set with all of the four strains in TSB with a known amount of elemental S at 28° C. for a period of up to 100 days. Production of sulfate sulfur from the elemental sulfur were measured at 0, 10, 20, 30, 40, 60, 80 and 100 day intervals (FIG. 1). The capabilities of sulfur oxidation by the strains RAY12, RAY28, RAY132 and RAY209 can also be seen in Table 2. It is interesting to note that RAY12, RAY132 and RAY209 oxidize 30-48% of elemental sulfur between 30 and 60 days when the canola plant needs the sulfur most.
example 3
Seed Germination / Emergence Test
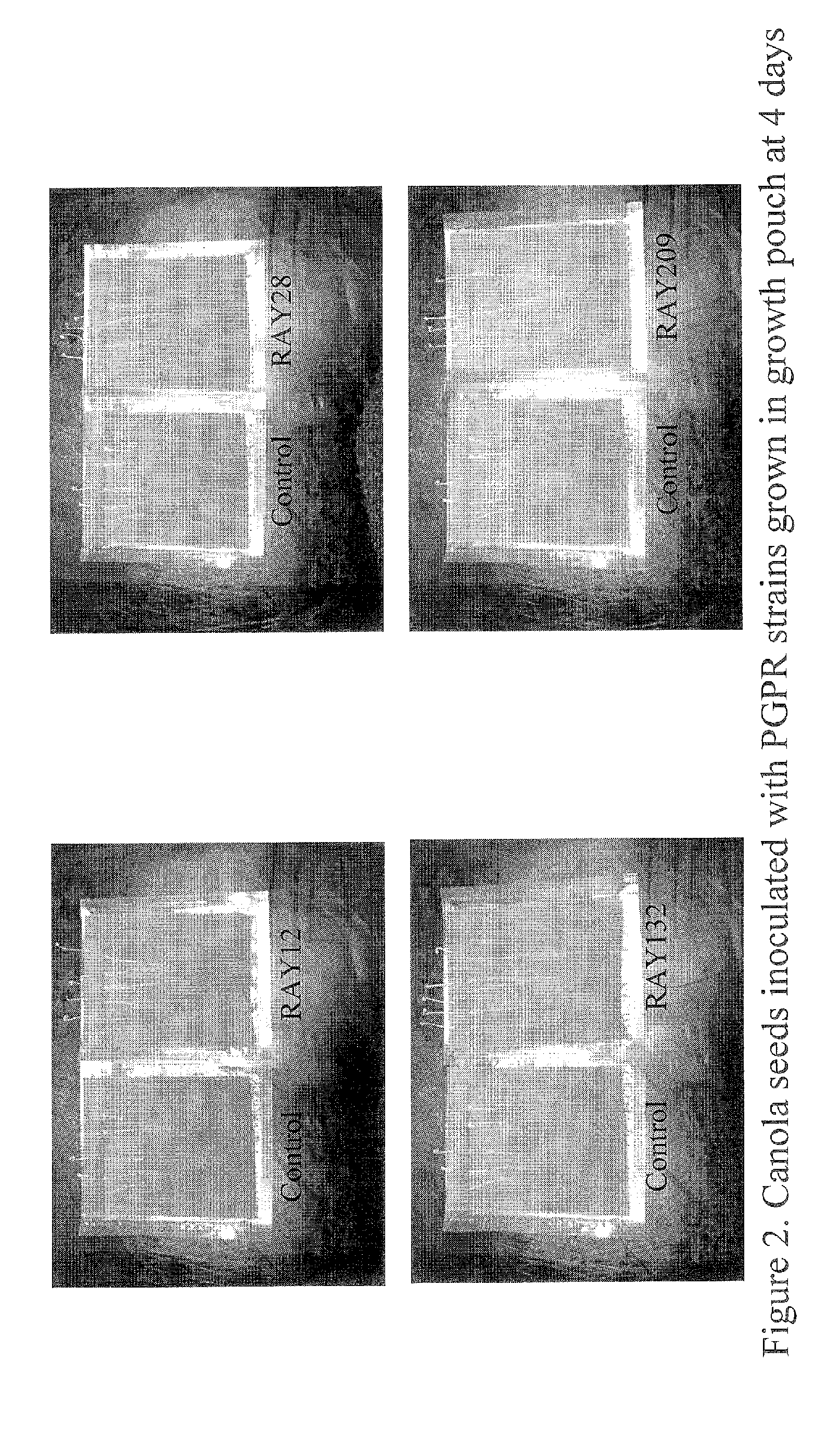
[0058] Herbicide-tolerant cultivar 799RR canola seed were surface sterilized for bacterial inoculation. Bacteria were grown in TSB for 48 hours and harvested by centrifugation. Bacterial numbers were determined by plating serial dilution of that washed cell cultures on TSA plates. Surfaced sterilized canola seeds were inoculated with the appropriate washed bacterial cultures and spread on to TSA plates to examine the effect on seed germination (Table 3). Sets of uninoculated seeds were also spread on the TSA plates as control (Table 3). Besides agar plates, seed germination and / or emergence test was also done in soil (Table 4) as well as using growth pouch (Table 5). Results indicated that none of the rhizobacterial isolates inhibited canola seed germination (Table 3). However, the bare canola seeds inoculated with bacterial isolates seem to accelerate germination time compared to control (Table 4 and Table 5).
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com