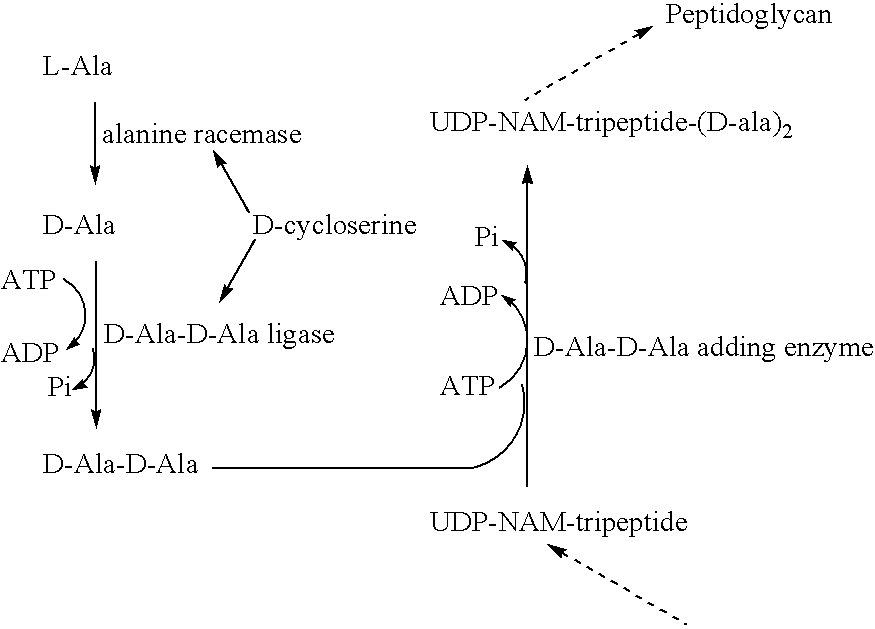
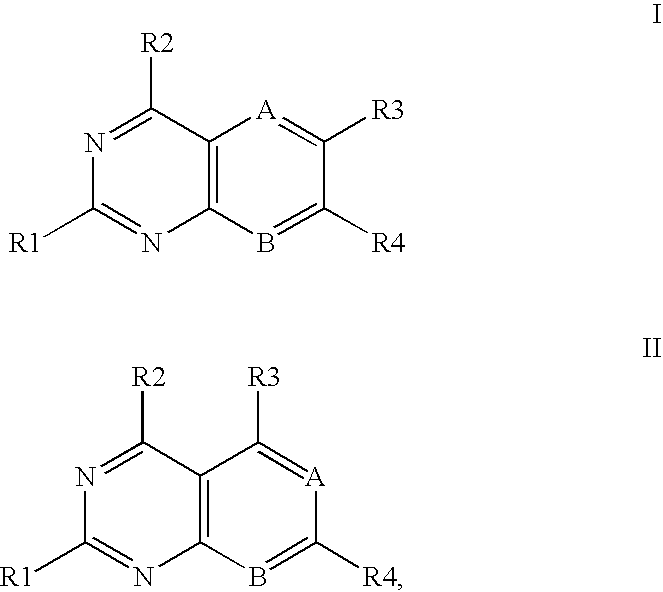
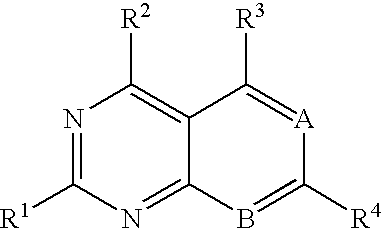
Heterocyclic compounds and uses thereof as d-alanyl-d-alanine ligase inhibitors
a technology of d-alanyl-d-alanine ligase and heterocyclic compounds, which is applied in the field of heterocyclic compounds, can solve the problems of increasing the resistance of organisms to the various exogenous factors, chronic infection or even death, and complex pathogenic processes by which microorganisms elicit adverse effects on subjects, etc., and achieves broad spectrum activity, reduced side effects, and different selectivity profiles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Ligase Inhibitors
[0136]
N7-Methyl-N-7-(1-naphthalen-1-yl-propyl)-pyrimido[4,5-d]pydrimidine-2,4,7-triamine
[0137] Compound 1: To a solution of 4.25 g (27.2 mmol) naphthaldehyde in 30 ml dry ether in ice water bath was slowly added 13 ml of ethylmagnesium bromide, 3 M in ether. The mixture was stirred for another 30 min at room temperature and then quenched by adding 40 ml of 1N HCl solution. The organic layer was washed with water (20 ml), sat. sodium bicarbonate (20 ml×2), brine (20 ml) and then dried over anhydrous sodium sulfate. Evaporation of the organic solvent gave a crude product 1 which was directly used for the next step of the reaction without further purification.
[0138] Compound 2: The crude product 1 was dissolved in 30 ml acetone and to the resulting mixture, bathed in an ice water bath, was slowly added Jone's reagent until the brown color persisted. The solution was further stirred for 15 min at room temperature and then 5 ml of isopropanol was added. A...
example 2
D-Ala-D-Ala-Ligase Ki Determination
[0181] The synthetic analogs of Example 1 were dissolved in dimethylsulfoxide (DMSO) at a concentration of 100 mM on the day of screening, using a vortex mixer and sonication if necessary for dissolution. The solutions were kept at room temperature until screening was completed.
[0182] A 10 mM NADH (Sigma) stock solution was prepared freshly on the day of screening by dissolving 32 μmol NADH in 3.2 ml double-distilled water. The NADH solution was kept on ice. Stock solutions containing 50 mM phosphoenolpyruvate (PEP; Sigma), 500 μM HERMES, 30 mM adenosine triphosphate (ATP; Sigma), 200 mM D-alanine (Sigma), and 4× core buffer (i.e., 400 mM Hepes, 40 mM magnesium chloride, and 40 mM potassium chloride), were also stored on ice. A stock solution of pyruvate kinase / lactate dehydrogenase (PK / LDH) was also obtained from Sigma.
[0183] Table of Final concentrations are dependent on the type of screening:
ANALOGS' %ANALOGS' Ki ANDANALOGS' KiINHIBITIONMOD...
example 3
Determination of Ki of Analogs
[0185] For each set of test compounds, two 96-well plates were used: an inhibitor plate and an enzyme plate. The test compounds correspond to rows A-G of the plates. Adenosine (Sigma) dissolved in DMSO, used as a control, corresponds to row H of each plate.
[0186] The enzyme solution was allowed to equilibrate to 25° C.
[0187] Dilutions were prepared in the inhibitor plate as follows: 50 μl DMSO was added to each well of columns 1-11, rows A-G, of the inhibitor plate. 50 μl DMSO were added to each well of columns 1-11, row H. 100 μl of the 100 mM test solutions were added to column 12, rows A-G (i.e., the first compound in row A, the second compound in row B, and so on). 100 μl of a 100 mM Adenosine solution was added to column 12, row H.
[0188] 50 μl of the test solution was transferred from column 12 in each row to column 11 of the a same row, mixing the solution with the DMSO. 50 μl of solution was then transferred from column 11 in each row to colu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| total volume | aaaaa | aaaaa |
| total volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com