NOx Storage Materials and Traps Resistant to Thermal Aging
a technology of storage materials and traps, applied in the direction of physical/chemical process catalysts, metal/metal-oxide/metal-hydroxide catalysts, separation processes, etc., can solve the problems of catalysts that use baria for nosub>x /sub>storage, nosub>x /sub>storage capacity loss, and ineffective twc catalysts for reducing nosub>x /sub>emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of NOx Storage Material
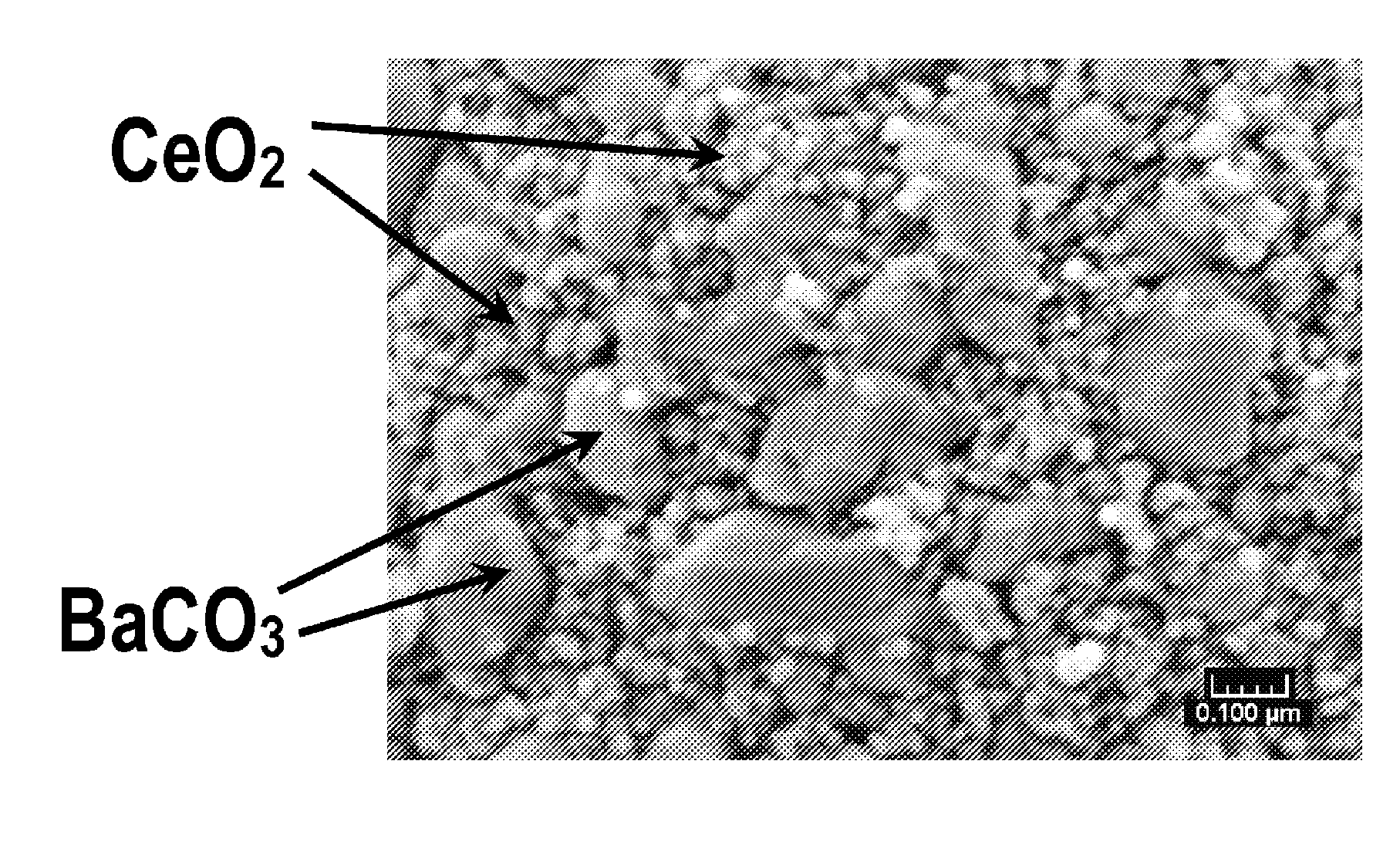
[0034]BaCO3 and CeO2 were intimately mixed and finely dispersed in a weight ratio of between about 1:3 and about 1:5. Cerium oxide having a BET surface area of between about 50-150 m2 / g was mixed with a solution of barium acetate such that the BaCO3 / CeO2 composite had a BaCO3 content of about 10-30 wt %. After mixing, the suspension of soluble barium acetate and CeO2 was then spray-dried at a temperature of between about 90° C. and 120° C. to obtain a solid mixture of barium acetate and ceria.
[0035]After spray-drying, the mixture was then heated at about 550° C. to 800° C. for about 2 hours to form particles of ceria having barium carbonate supported on the ceria particles. The resulting BaCO3 had a crystallite size of between about 20 and 40 nm. The BaCO3 and CeO2 crystallites formed particles with a size of between about 5 and 50 microns. The BET surface area of the particulate mixture is between about 30 and 80 m2 / g.
Preparation of Catalytic Comp...
example 3
NOx Storage Capacity Testing
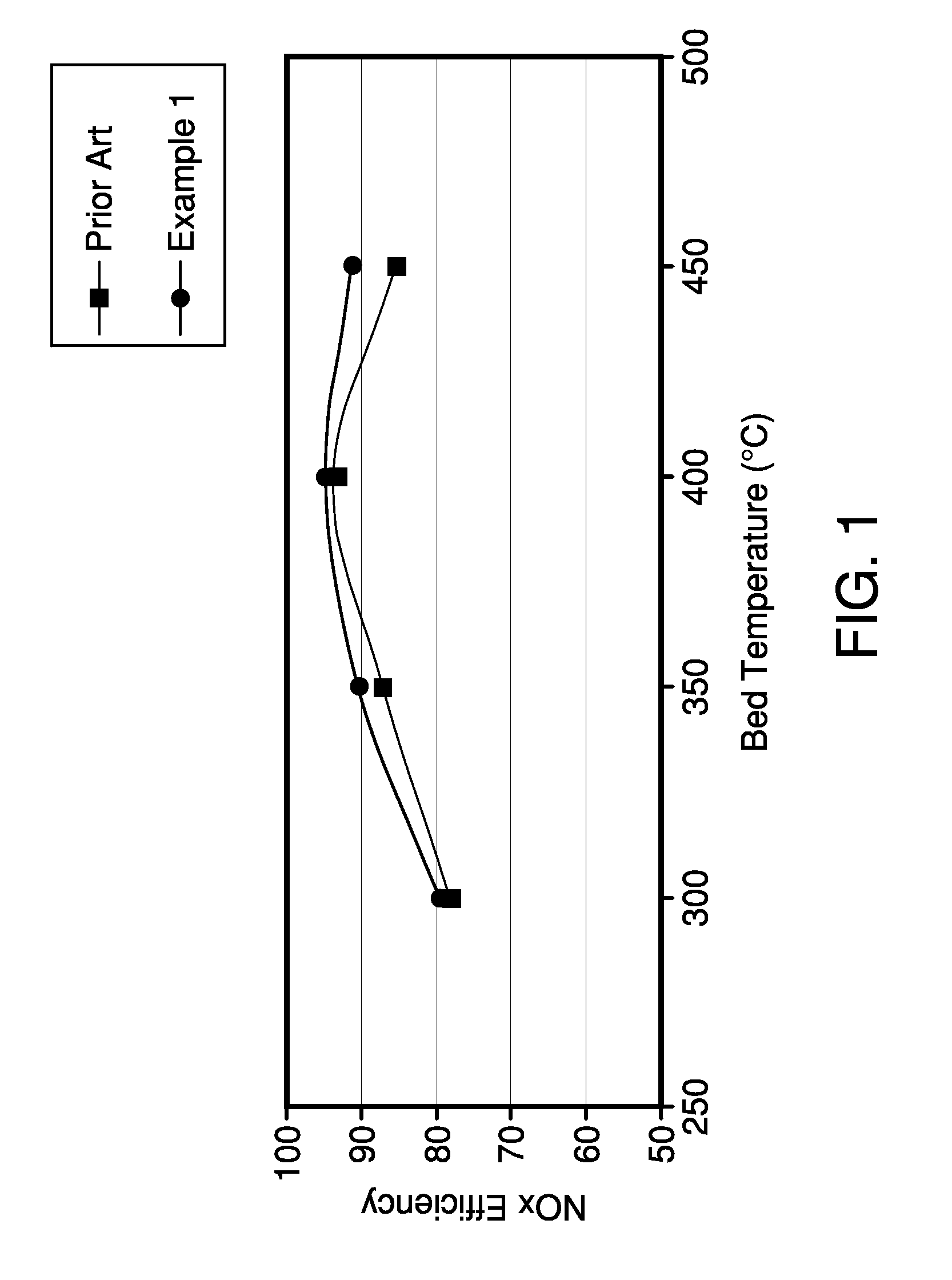
[0040]Two catalytic traps were prepared, a first catalytic trap was prepared in accordance with Example 1 and a comparative catalytic trap was prepared in accordance with Comparative Example 2. Both catalytic traps A were evaluated after aging for 8 hours at 850° C.
[0041]Both catalytic traps were evaluated as follows. An engine was set to an air / fuel ratio of 11.6 for 2 minutes at the desired temperature to remove all stored NOx and oxygen from the catalyst. This mode represents rich engine operation. Subsequently, the engine was adjusted to an air / fuel ratio of 29.6 under constant NOx mass flow. This mode represents lean engine operation. During the whole test, the NOx concentration was measured before and after the NOx trap using a NOx analyzer.
[0042]After the 2 minute rich operation followed by a 60 second lean operation, the engine was set to a 3 second rich operation to remove stored NOx without having hydrocarbon and carbon monoxide tailpipe emissio...
example 4
Barium Concentration and Calcination Temperature
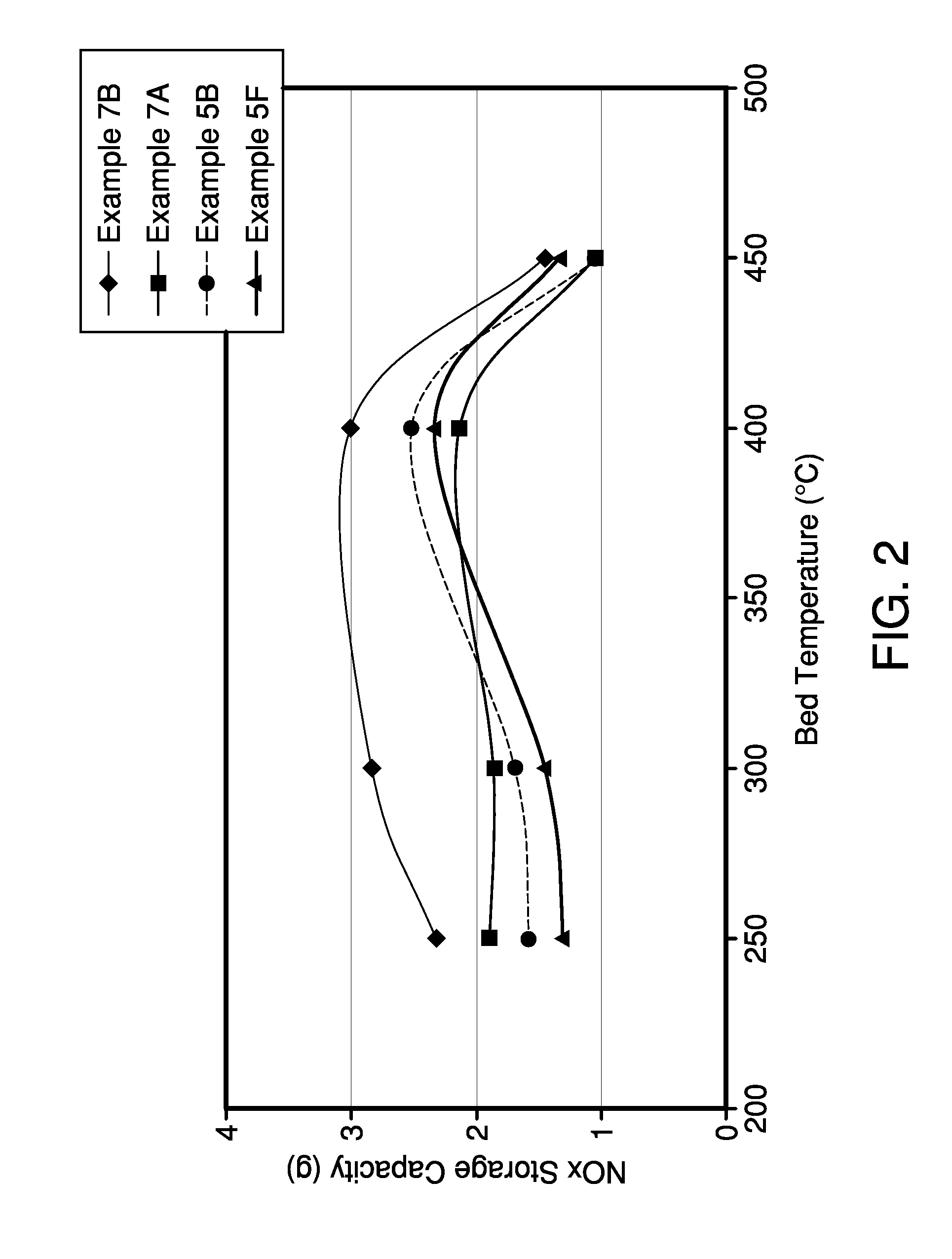
[0049]Different amounts of Ba were impregnated into ceria of different surface area, using the procedures described in Example 1. Ceria powders with different BET surface areas were used to determine the effect of the resulting Ba / Ceria composite powder.
[0050]Characterization of the impregnated powder included BET surface area measurement. In addition fully formulated NOx trap catalysts were prepared using the procedures described in Example 1 that contain the particular Ba / Ceria composite material as NOx storage component. The NOx storage properties of the catalysts have been evaluated after aging for 8 hours at 850° C. under air with 10% H2O in a laboratory reactor. The results are shown in Table I and Table II below.
[0051]Table I shows the result of a variation of the BaCO3 and CeO2 concentration together with a variation of the ceria used. After impregnation, all samples were calcined at 550° C. in air to decompose the impregnated ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com