Intravascular folded tubular endoprosthesis
a folded tubular endoprosthesis and tubular technology, applied in the field of vascular implants, can solve the problems of high morbidity and mortality, onset of accompanying symptoms and requiring repair, and vascular tubular dislocation, so as to reduce the likelihood of distal migration of the vascular tubular member, reduce the chance of thrombosis formation, and ensure the effect of vascular integrity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
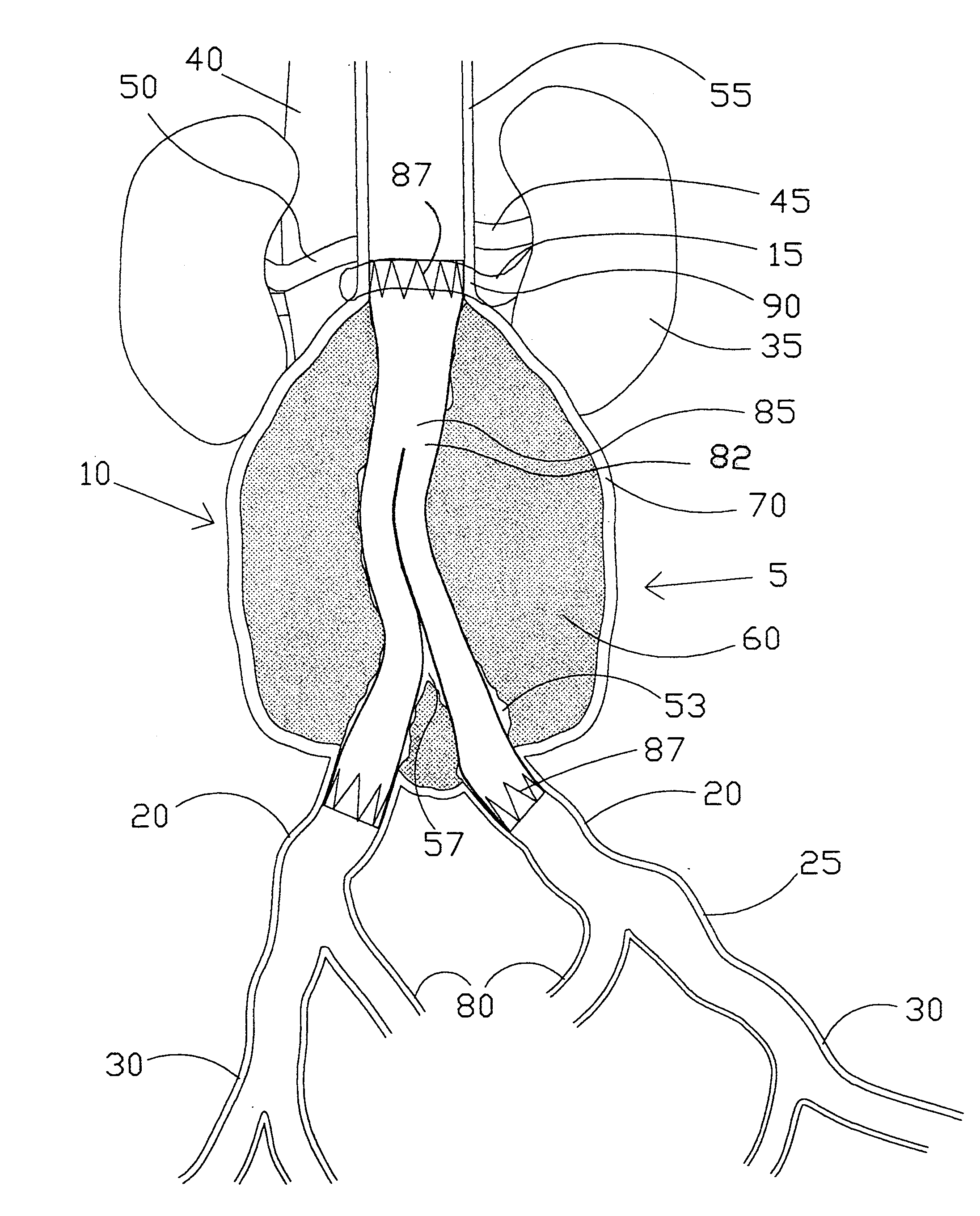
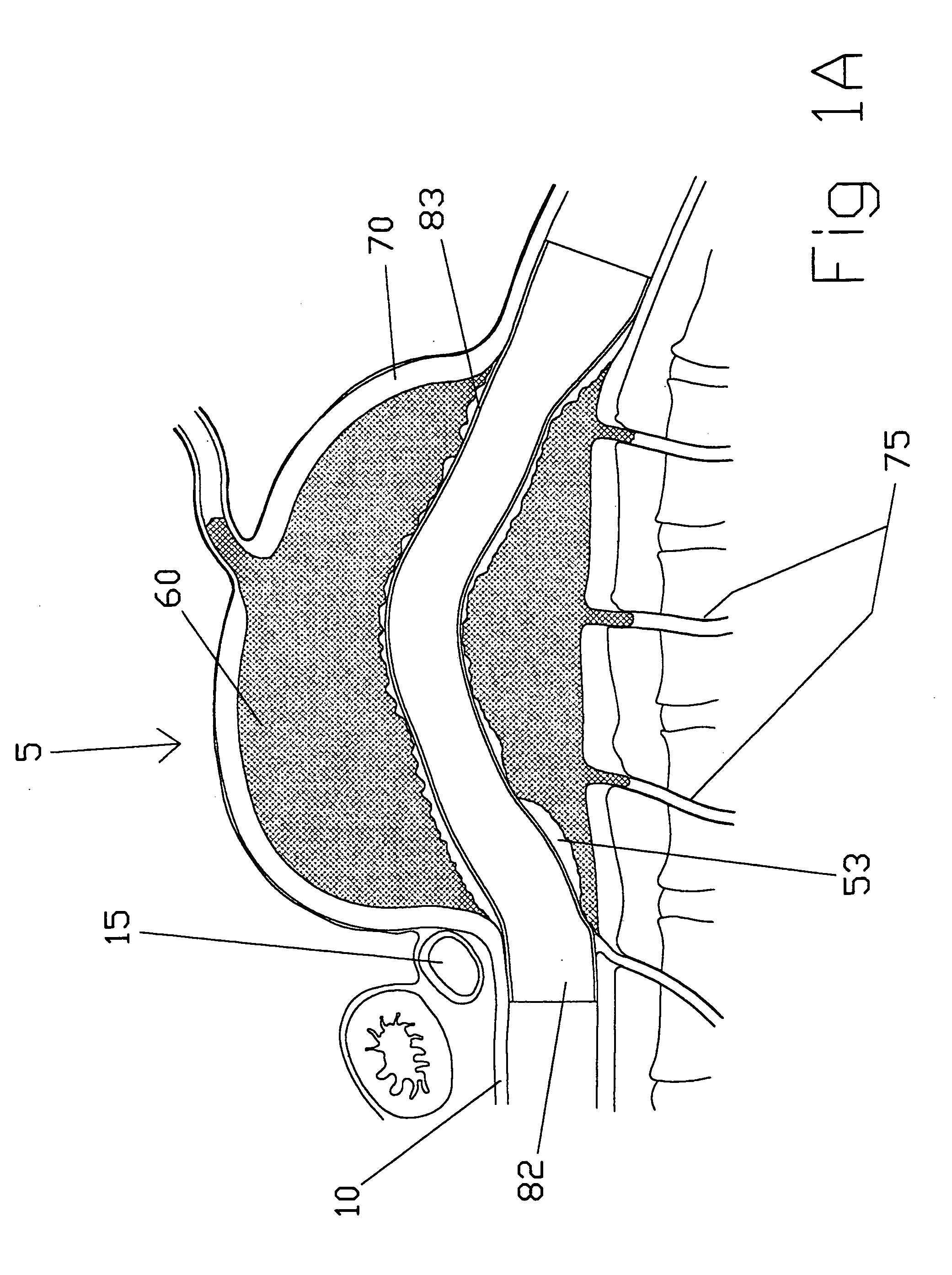
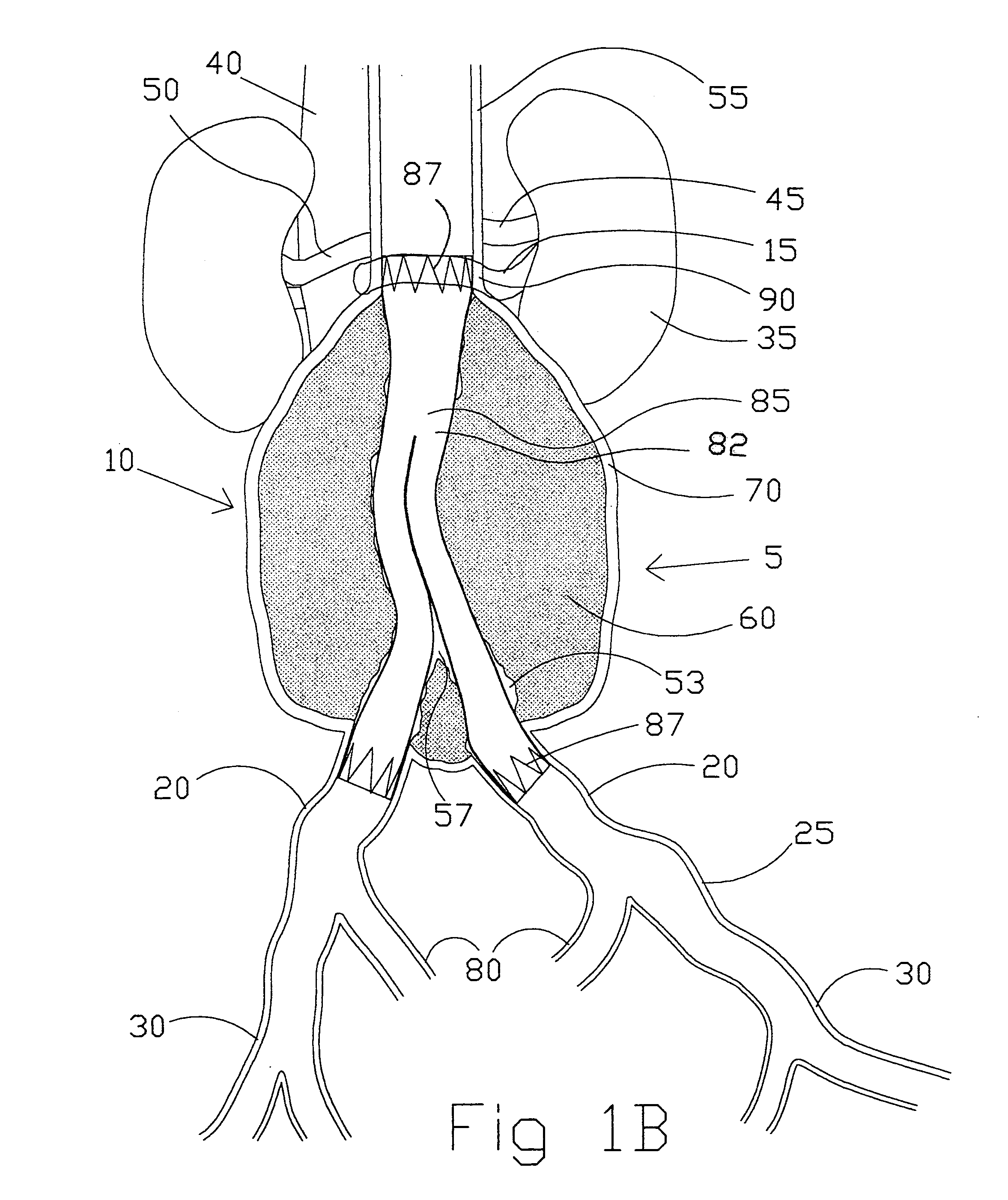
[0123]the present invention (see FIGS. 2A-2D) is a straight intravascular folded tubular member 95 for repairing an arterial lesion, an aneurysm, or other vascular injury found in a blood vessel. The straight intravascular folded tubular member 95 is intended to provide a blood flow passage 100 from a region of the blood vessel proximal to the vascular injury to a region of blood vessel distal to the vascular injury. The preferred method of deploying the straight intravascular folded tubular member 95 is to insert it through a percutaneous access through a sheath as is well known in the industry or with a small surgical cutdown to provide direct access to a blood vessel located either proximal or distal to the vascular injury.
[0124]FIG. 2A shows the straight intravascular folded tubular member 95 in a radially deployed state with a radially deployed inlet end diameter 105, a radially deployed outlet end diameter 110, and a straight nondeployed tubular member length 115. The straight...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com