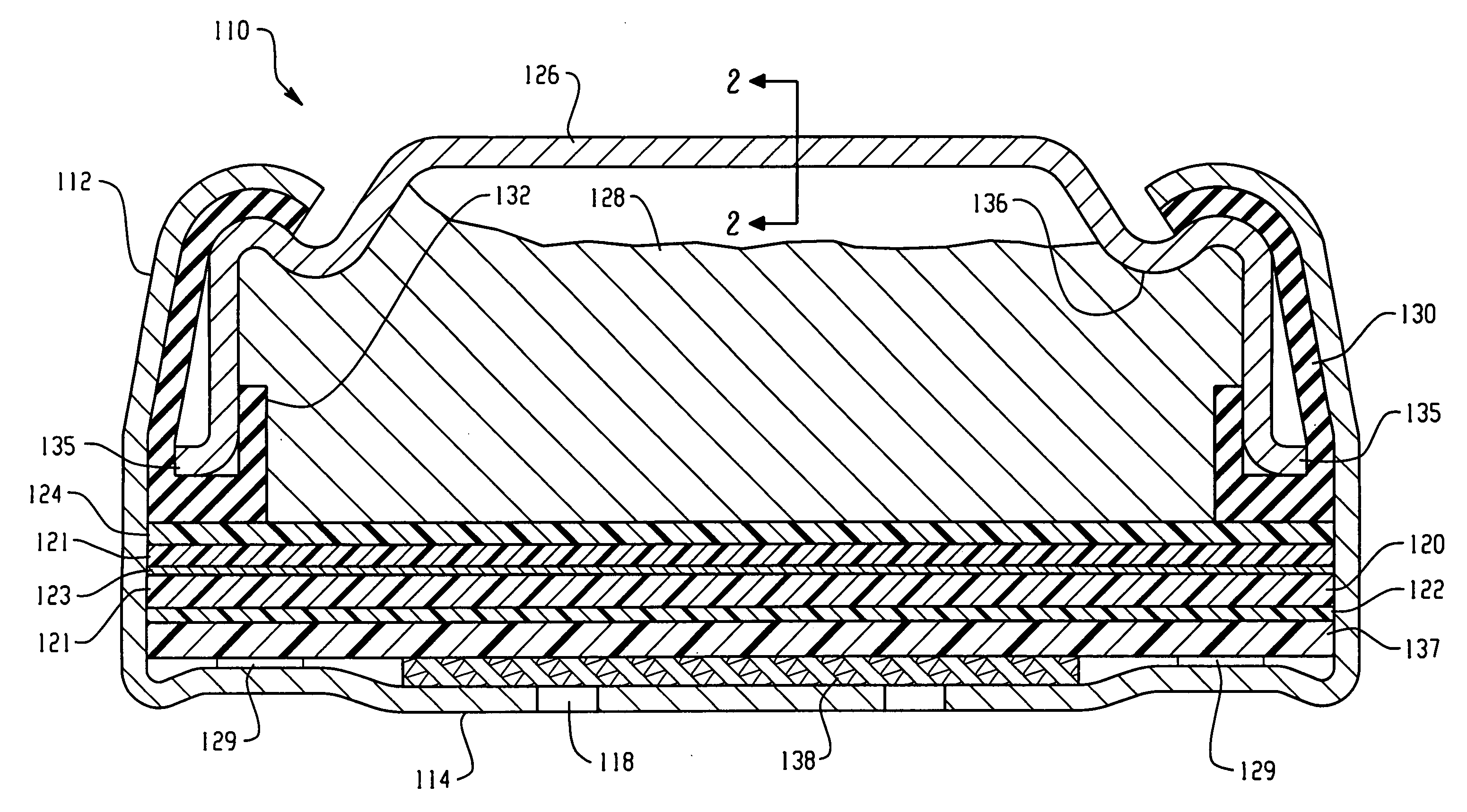
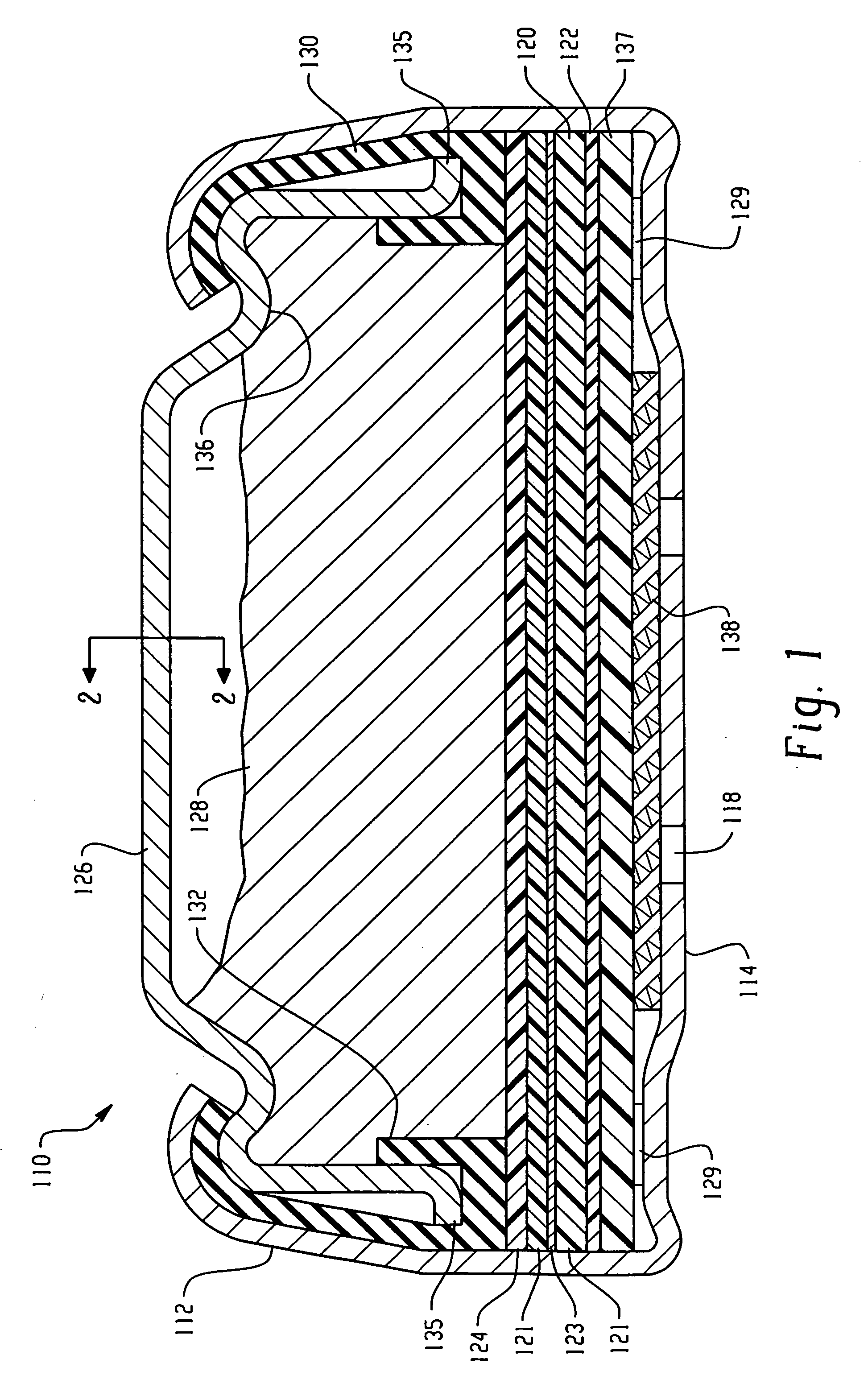
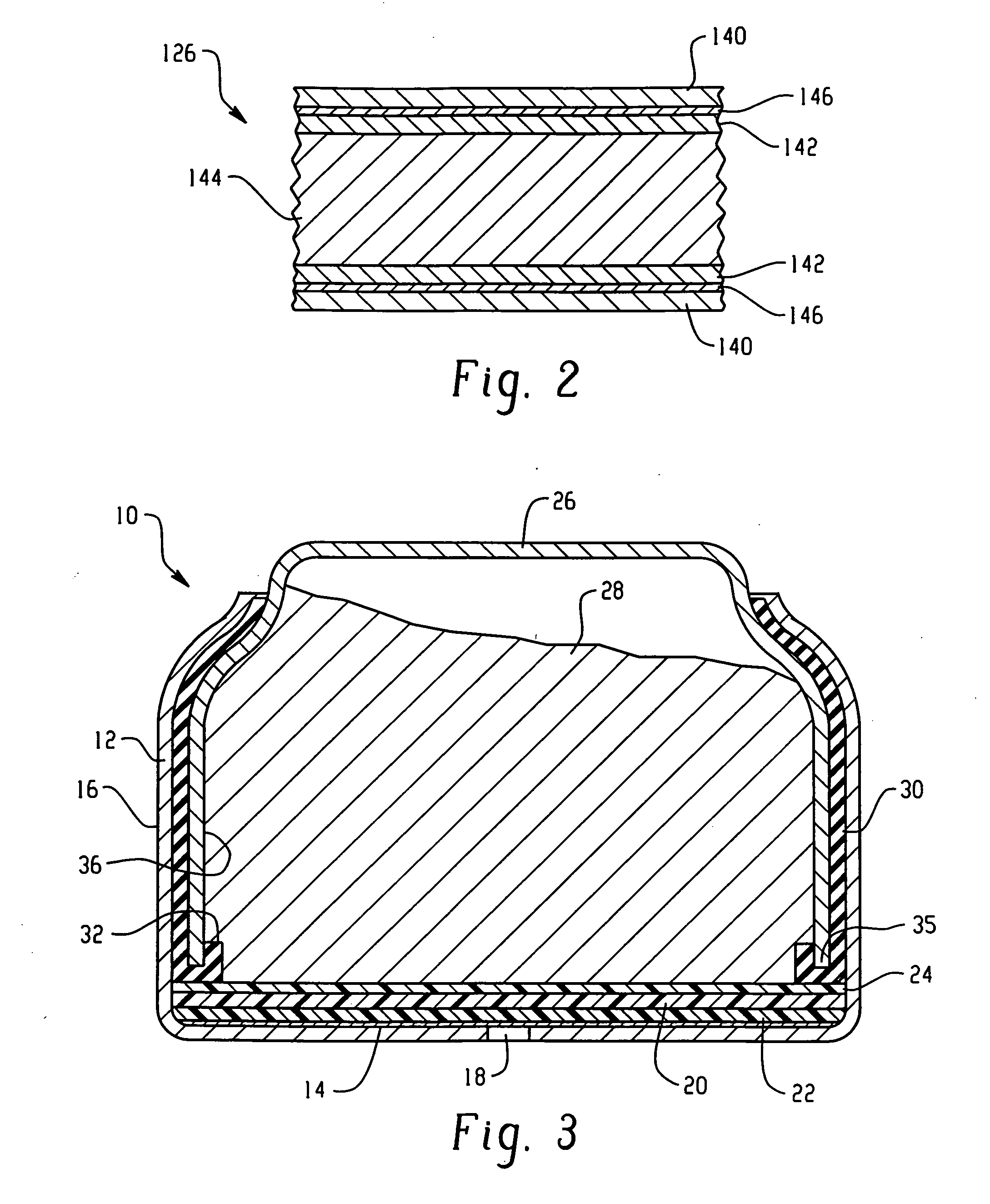
Electrochemical Cell With A Catalytic Electrode And Process For Making The Electrode And The Cell
a catalytic electrode and electrochemical cell technology, applied in the field of catalytic electrodes, can solve the problems of placing restrictions and adding costs to the manufacturing process that can be undesirable, and achieve the effect of convenient, economical and safe manner
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
[0100]A nano-catalyst air electrode was made with nano-MnOx according to the following steps:[0101]1. 300 g DARCO® G-60 carbon was placed in a 2000 ml glass container, 1000 g distilled water was added, and the container was covered and allowed to sit for one hour.[0102]2. The mixture was mixed for about 30 minutes with an adjustable speed mixer, adjusting the speed to maintain a vortex extending about half way to the bottom of the container.[0103]3. 55 g T30B PTFE was added dropwise while mixing, and mixing was continued for about 20 minutes, adjusting the speed to maintain the desired vortex.[0104]4. The mixture was filtered, while rinsing with about 200 ml distilled water.[0105]5. The remaining solids (the mix) were dried at about 85-90° C. for about 16 hours, then the temperature was increased to 105° C. and drying continued until the temperature of the mix reached 105° C.; then the mix was covered and allowed to cool to room temperature.[0106]6. In an argon gas atmosphere, 10 gr...
example 3
[0113]A sheet of BVA 02530 separator (Hollingsworth & Vose) was glued to the electrode sheets from each of Examples 1 and 2, on the surfaces of the air electrodes opposite the PTFE membranes, using pressure and a PVA / CMC adhesive. Sample electrodes were cut from each of the sheets and assembled into PR44 size alkaline zinc-air button cells.
[0114]Cells with electrodes from each of Examples 1 and 2 were tested for open circuit voltage, followed by AC impedance, with a peak to peak potential amplitude of 10 mV, over a frequency range from 65 KHz to 0.1 Hz. This was followed by a potential dynamic scan in the cathodic direction, beginning at 0.025 V above the open circuit voltage and scanning at 1 mV / sec. to 0.7 V. This initial AC impedance and potential dynamic scan testing served to condition the catalytic electrodes. After 30 minutes open circuit, the AC impedance and potential dynamic scan tests were repeated, and these results were used. From the potential dynamic voltage scan, a v...
example 4
[0116]A nano-catalyst air electrode was made with nano-MnOx according to the following steps:[0117]1. 200 g DARCO® G-60 carbon was placed in a 1500 ml glass container, 900 g distilled water was added, and the container was covered and allowed to sit for about 15 minutes.[0118]2. The mixture was mixed for about 15 minutes with an adjustable speed mixer, adjusting the speed to maintain a vortex extending about half way to the bottom of the container.[0119]3. Mixing was continued while 5.6 g nano-MnOx (QSI-NANO® Manganese) was slowly (over about 30 seconds) added to the vortex and the sides of the container were rinsed with deionized water, followed by about 15 minutes of additional mixing, adjusting the mixer speed to maintain the desired vortex.[0120]4. Mixing was continued while 26 g of T30B PTFE was added dropwise to the vortex, followed by about 20 minutes of additional mixing, adjusting the mixer speed to maintain the desired vortex.[0121]5. The mixture was filtered, while rinsin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| BET specific surface area | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com