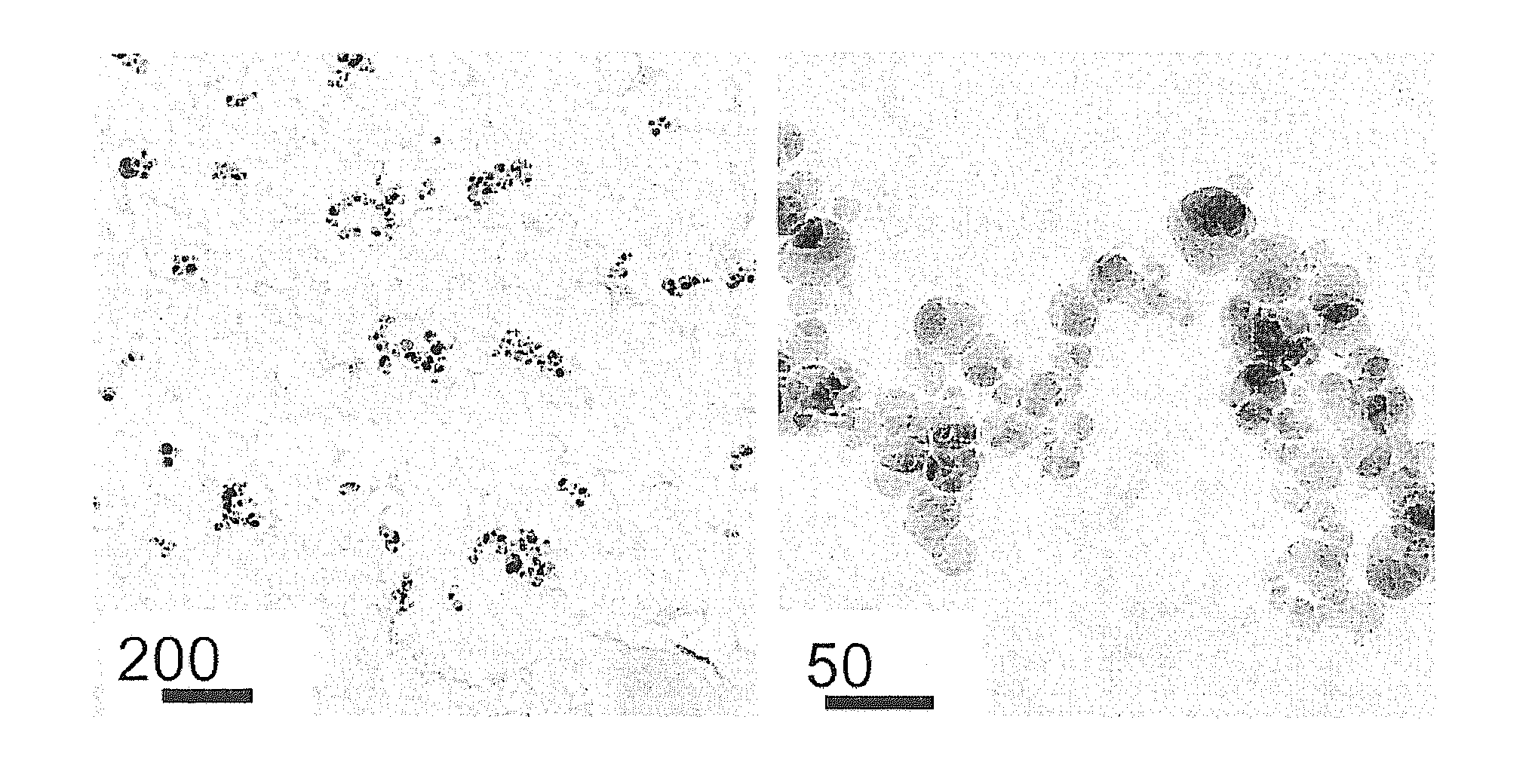
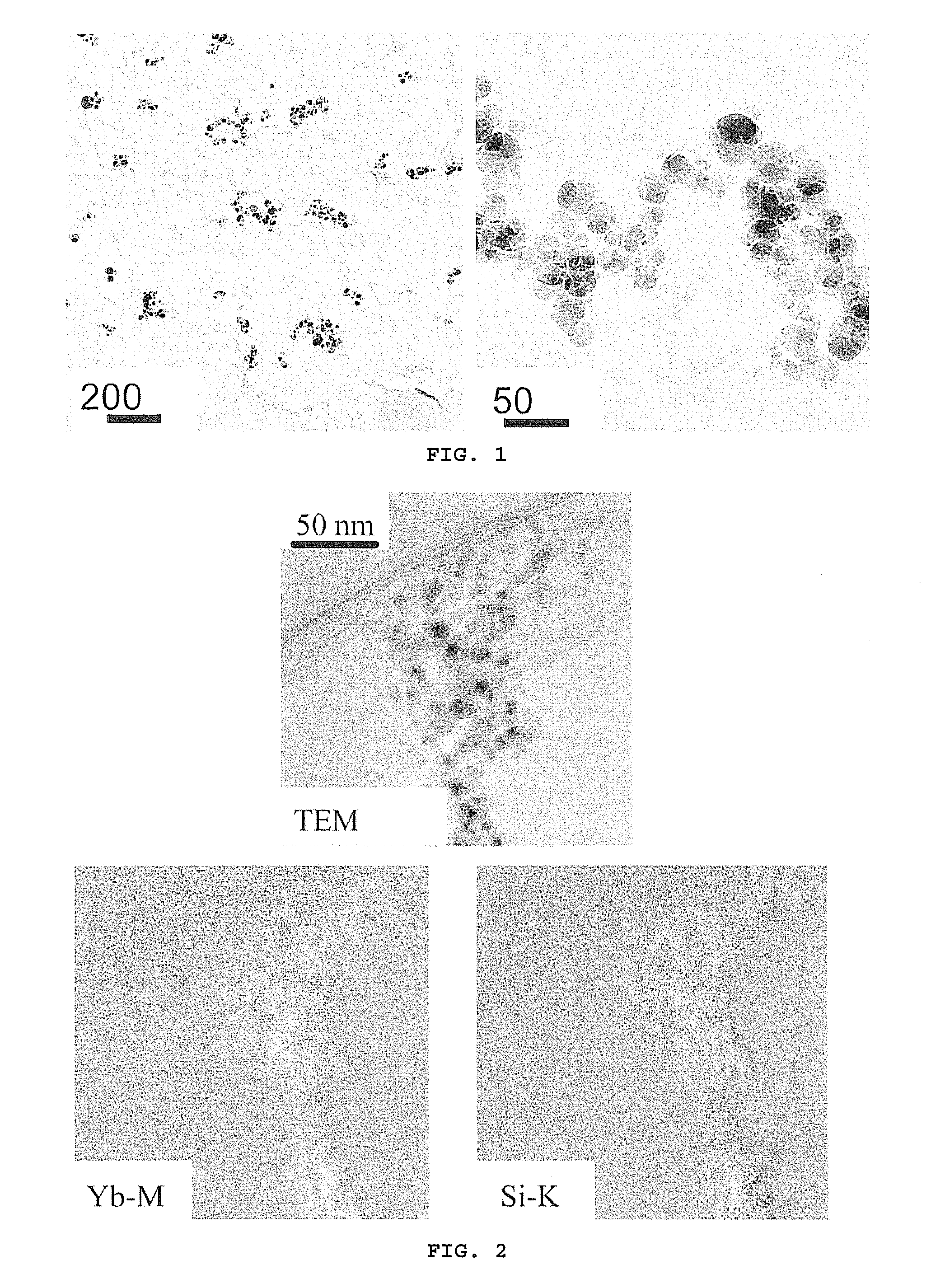
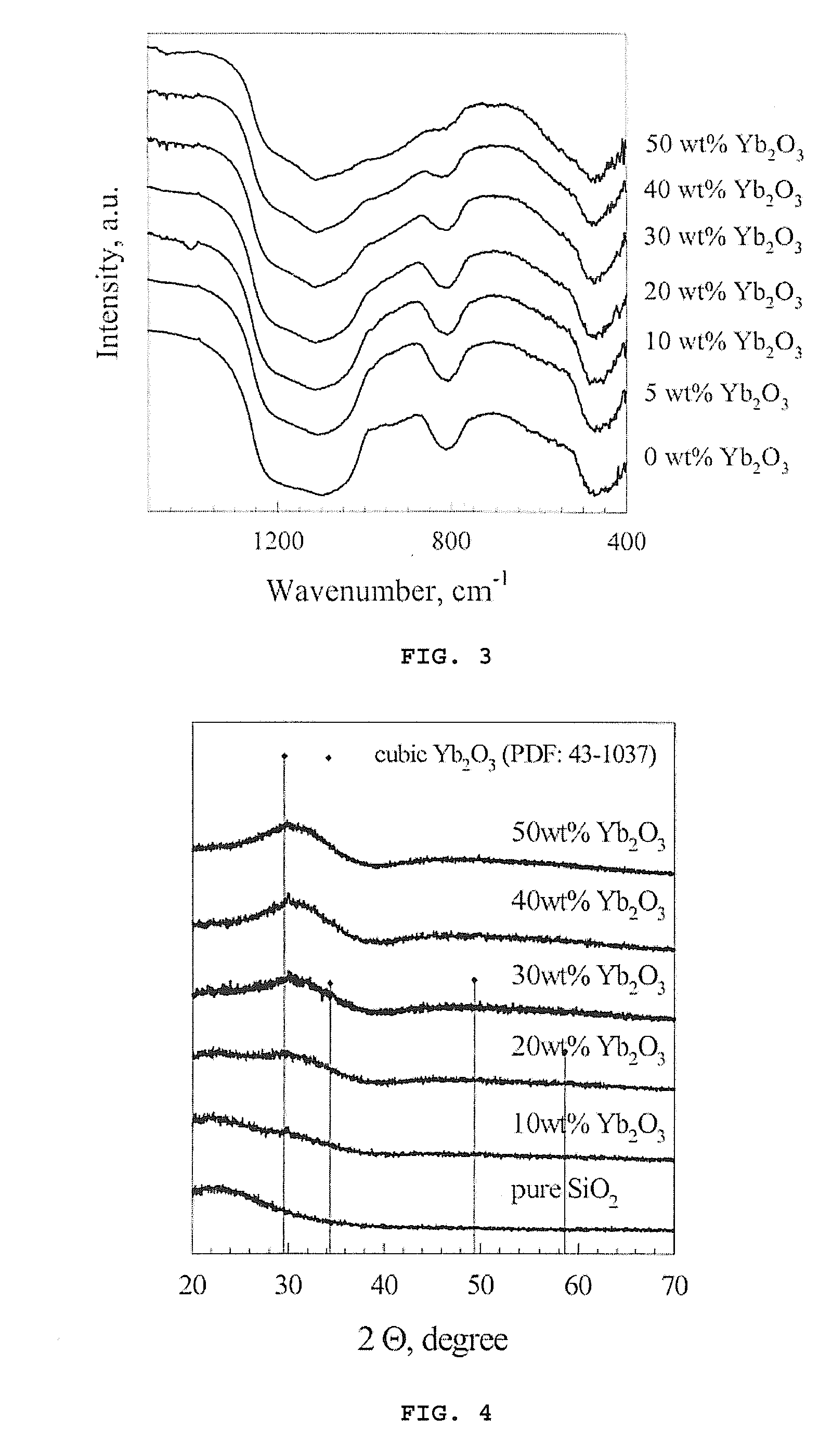
Dental composites based on x-ray-opaque mixed oxides prepared by flame spraying
a technology of mixed oxides and dental composites, which is applied in the field of dental composites based on x-ray-opaque mixed oxides prepared by flame spraying, can solve the problems of reduced transparency and aesthetics, high cost, and high cost of dental composites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Mixed Oxides of the Elements Si and Yb
[0078]A two-fluid nozzle was used at a liquid inflow rate of 5 ml / min. The atomization gas was oxygen (5 l / min). The support flame was operated using premixed methane / oxygen (1.5 l / min / 3.2 l / min). The enveloping air stream was 5 l / min of oxygen. The precursors used for Si and Yb were tetraethoxysilane (TEOS) and ytterbium nitrate pentahydrate (Yb (NO)3.5H2O), respectively. To remove all water of crystallization from the Yb precursor, Yb(NO)3.5H2O was reacted in 18.75% by volume of acetic anhydride and 81.25% by volume of 2-ethylhexanoic acid at 107° C. under inert gas (N2). In this reaction, all oxides of nitrogen were driven off and the water was removed by reaction with acetic anhydride to form acetic acid. The solution formed in this way was mixed with 45.08% by volume of xylene and TEOS to give a total metal concentration of 0.5 mol / l. Various Yb / (Yb+Si) ratios could easily be set using this method. Solutions containing up to 50...
example 2
Evaluation of the Thickening Action of the Mixed Oxides of the Elements Si and Yb Prepared as Described in Example 1
[0081]To examine the thickening action, model composite pastes were prepared from 16.5% of Yb / Si mixed oxide of differing specific surface areas and 83.5% of a free-radically polymerizable monomer composition (41.82 parts of bis-GMA, 37 parts of UDMA, 20 parts of TEGDMA, 0.73 part of photoinitiator, 0.55 part of additives). 0.1 g of paste was loaded between two glass plates under a load of 120 g for a period of three minutes and the resulting diameter of the paste was determined. The thinner this “disc consitency” of the paste, i.e. the lower the thickening action, the greater the diameter obtained. The results demonstrate that the consistency of the pastes depends significantly on the specific surface area of the nanoparticle filler (Table 3).
TABLE 3Influence of the specific surface area of themixed oxide filler on the disc consistency of thecomposite pastesSpecific s...
example 3
Production of a Filling Composite Based on a Mixed Oxide of the Elements Si and Yb Prepared as Described in Example 1
[0083]A composite (composite A) was produced from 48% by mass of a light-curing monomer composition (41.82 parts of bis-GMA, 37 parts of UDMA, 20 parts of TEGDMA, 0.73 part of photoinitiator, 0.55 part) and 52% by mass of an X-ray-opaque Yb / Si mixed oxide having a Yb2O3 content of 30% by mass and a specific surface area of 125 m2 / g which had been prepared by flame spraying as described in Example 1. As a comparative example, a composite (composite B) was produced from 50% by mass of the same light-curing monomer, 35% by mass of silanized pyrogenic SiO2 OX-50 and 15% by mass of ytterbium fluoride, and the transparency and X-ray opacity were determined after curing of the composites:
TABLE 4Transparency and X-ray opacity of thecomposites from Example 3TransparencyMaterial(%)X-ray opacity (% Al)Composite A13.2180Composite B9.5150
[0084]The results show that an acceptable t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallinity index | aaaaa | aaaaa |
| crystallinity index | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com