Stationary capillary electrophoresis system
a capillary electrophoresis and capillary technology, applied in the direction of liquid/fluent solid measurement, fluid pressure measurement, peptide, etc., can solve the problems of difficult analysis of macromolecules in complex mixtures, affecting the growth of process bacteria, and the associated sampling of an operating bioreactor with numerous problems, so as to reduce the need for robotic system calibration and provide durability. , the effect of rapid sampling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0045]A description of preferred embodiments of the invention follows.
[0046]The methods and apparatus disclosed herein are generally related to analyzing a sample of a molecular analyte, e.g., a macromolecule, from a complex liquid mixture. The invention has particular application to automated methods and apparatus for capillary electrophoretic analysis macromolecules, e.g., proteins, from a complex bioreactor liquid mixture.
Automated Macromolecule Preparation
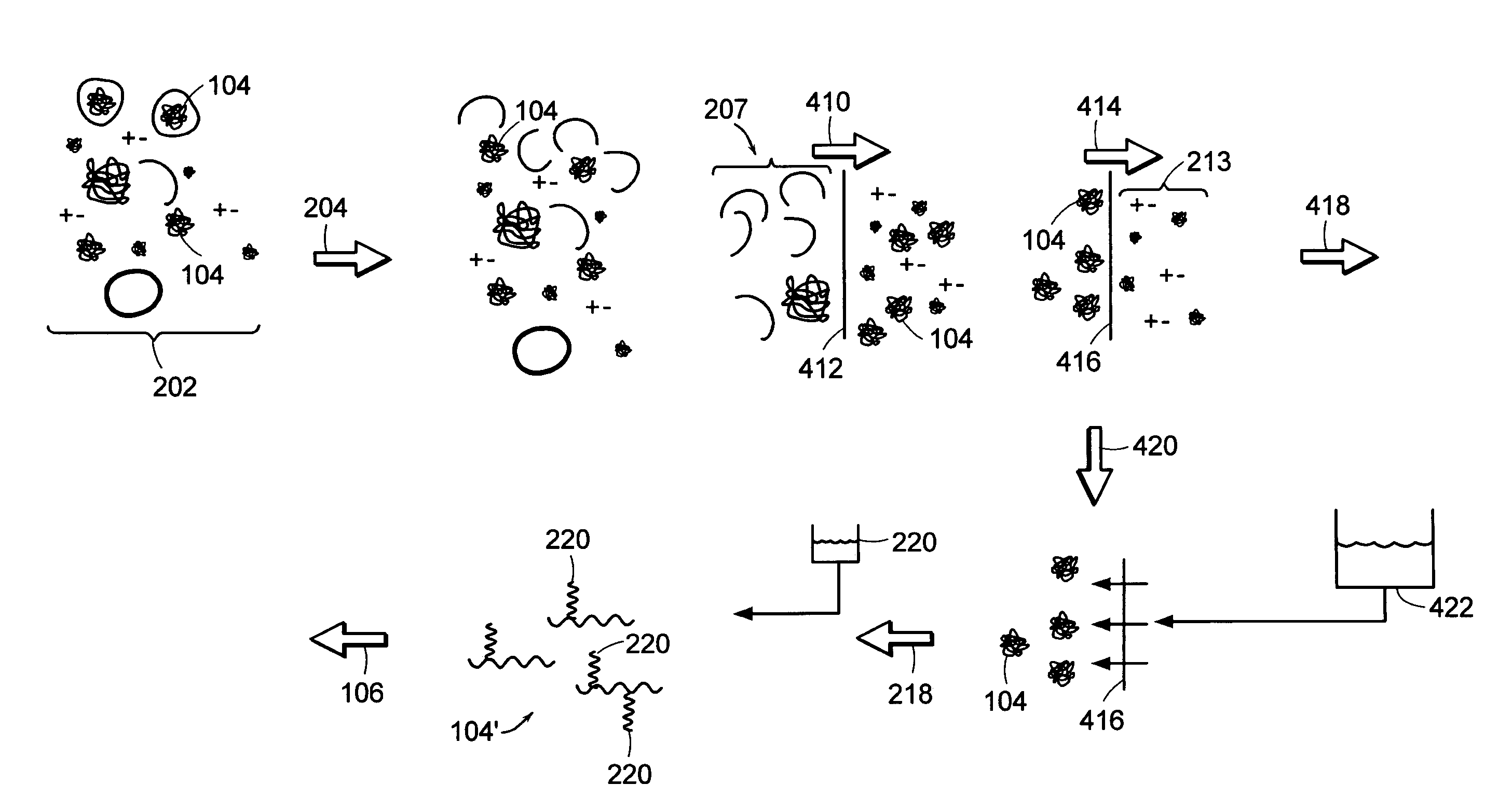
[0047]FIG. 4 depicts a schematic of steps that can be included in preparing a macromolecule sample. The liquid, typically aqueous, mixture 202 contains the macromolecule 104, and can also contain fine components 213, e.g., salts, molecules smaller than the macromolecule, and the like; and rough components 207, e.g., cells, cell fragments, particulate contaminants, molecules larger than the macromolecule, and the like.
[0048]Macromolecule 104 can be dissolved in the liquid mixture, or can be partially contained in cells, as depic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com