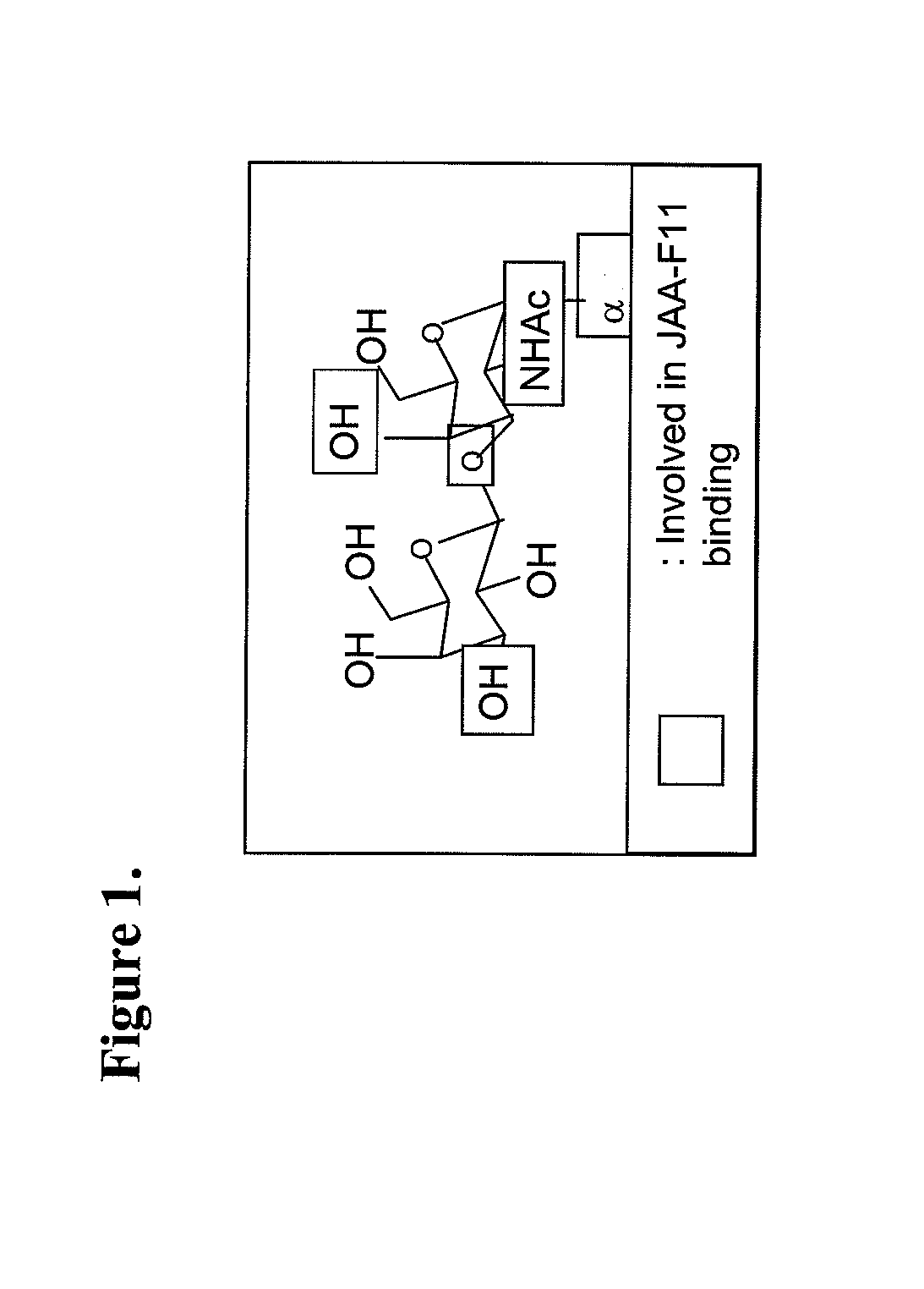
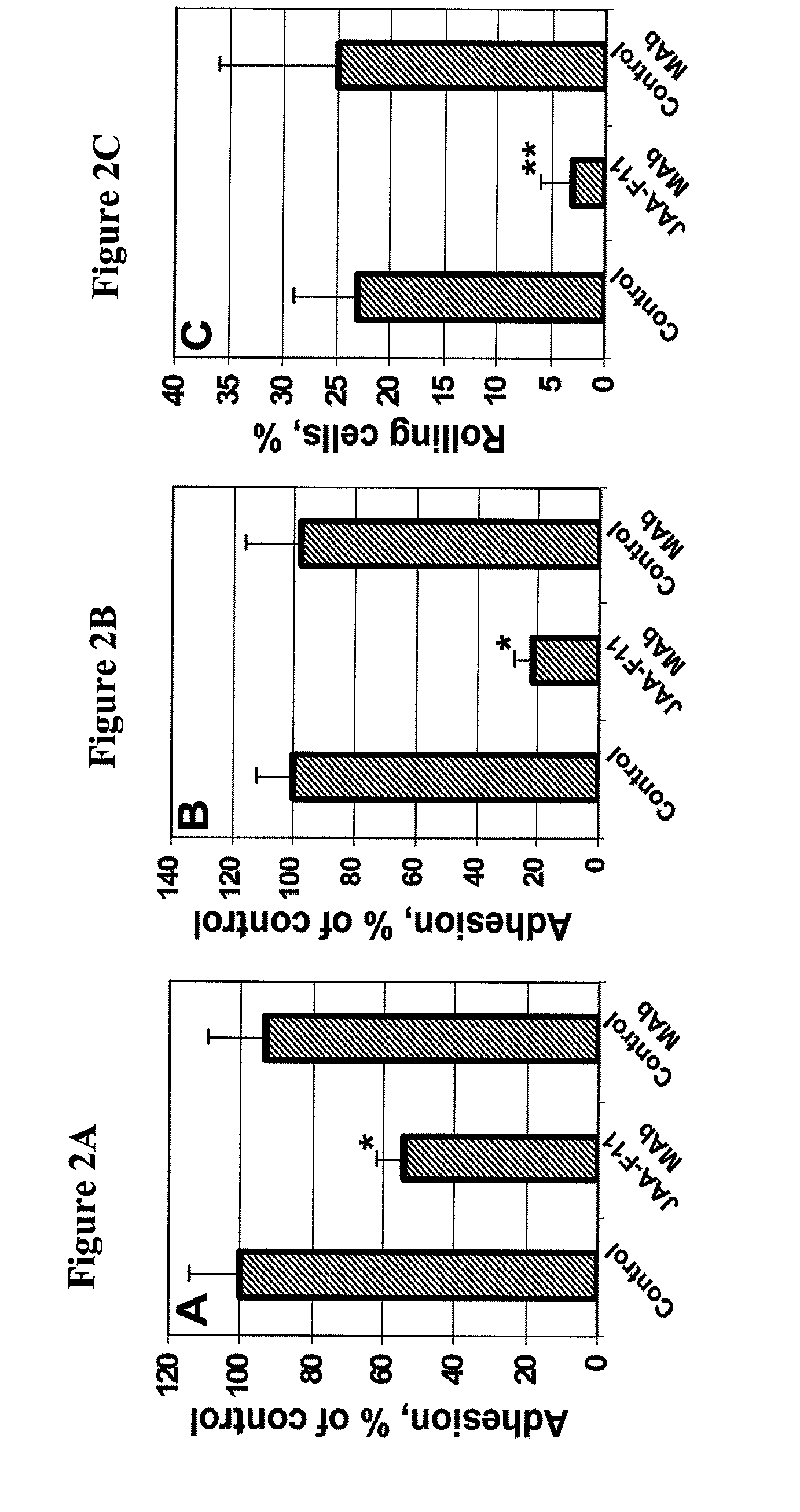
Therapeutic use of Anti-tf-antigen antibody
a technology of anti-tf antibody and therapeutic use, which is applied in the field of inhibition of cancer cell growth and metastasis, can solve the problems of limiting the potential use of anti-tf antibody, inability to dissolve at high concentration in aqueous buffer, and inability to cure cancer cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0041]This Example demonstrates the inhibition of cancer cell adhesion to endothelial and bone marrow cells by monoclonal antibody JAA-F11. Human umbilical vein endothelial cells (HUVEC) were purchased from Cascade Biologics (Portland, Oreg.). The basal Medium 200 (Cascade Biologics) supplemented with low serum growth supplement (LSGS) containing FBS (2% v / v final concentration), hydrocortisone, human fibroblast growth factor, heparin, and human epidermal growth factor was used for the HUVEC. The cells at passages 8-12 were used for the adhesion experiments. The human bone marrow endothelial cell line, HBMEC-60, provided by Dr. C. E. van der Schoot (University of Amsterdam, Amsterdam, the Netherlands), was developed by immortalization of HBMEC originally isolated from adult human bone marrow using the amphotrophic helper-free retrovirus pLXSN16 E6 / E7 (32). The HBMEC-60 cells were shown to maintain their normal phenotype and adhesive properties, specifically the ability to bind haema...
example 2
[0043]This Example demonstrates that monoclonal antibody can block a key stage of metastasis and metastatic tumor formation in an ex vivo mode. To perform these experiments, perfused porcine dura mater was used in adhesion experiments as previously described (33-34). Briefly, dura mater corresponding to one hemisphere, collected from mature female Yucatan miniature swine (Charles River, Me.) within 30 min after animals sacrifice in accordance with the University of Missouri approved animal care protocol, was dissected and flattened onto a Sylgard-coated 100 mm dish. A major branch of the median meningeal artery was cannulated, and dura vasculature was perfused at 15 μl / min first with Krebs physiological salt supplemented with 1.0 mg / ml porcine serum albumin for 20 min, then with vessel-labeling solution (0.3 μg / ml acridine orange in RPMI-1640 supplemented with 10% FBS and 1.0 mg / ml porcine albumin) for an additional 40 min. Prior to injection, cancer cells were pre-labeled for 5 min...
example 3
[0045]This Example demonstrates TF—Ag detection on tumor cell lines with JAA-F11 using Indirect Cellular ELISA. To perform these experiments, five mouse cancer cell lines were tested. 4T1 (ATCC Number: CRL-2539) and JC (ATCC Number: CRL-2116) are both from BALB / c strain mammary gland adenocarcinomas. The 4T1 cell line is an animal model for stage IV human breast cancer (35-37). When injected into BALB / c mice, 4T1 spontaneously produces highly metastatic tumors that can metastasize to the lung, liver, lymph nodes and brain while the primary tumor is growing in situ (35-37). JC also are able to produce tumors in BALB / c mice (38). Myeloma (ATCC Number: CRL-1580) is the fusion partner for producing JAA-F11 hybridoma (21), and was used as a TF—Ag-negative control cell line. LL (ATCC Number: CRL-1642) is the Lewis lung carcinoma cell line also from mouse (39-40). The RIF cell line is a radiation-induced fibrosarcoma. Except for the culture medium for LL (Dulbecco's modified Eagle's medium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com