Procedure for preparing redox-active polymers on surfaces
a technology of redox-active polymers and surface preparation, applied in the field of molecular electronics, can solve the problems of affecting the quality of redox-active molecules, requiring high concentration, and/or using reactive intermediates, and achieving failures, failures, and failures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cyclic Voltammetry And Coverage Summary of Redox-Active Molecules Containing Ethynyl Groups
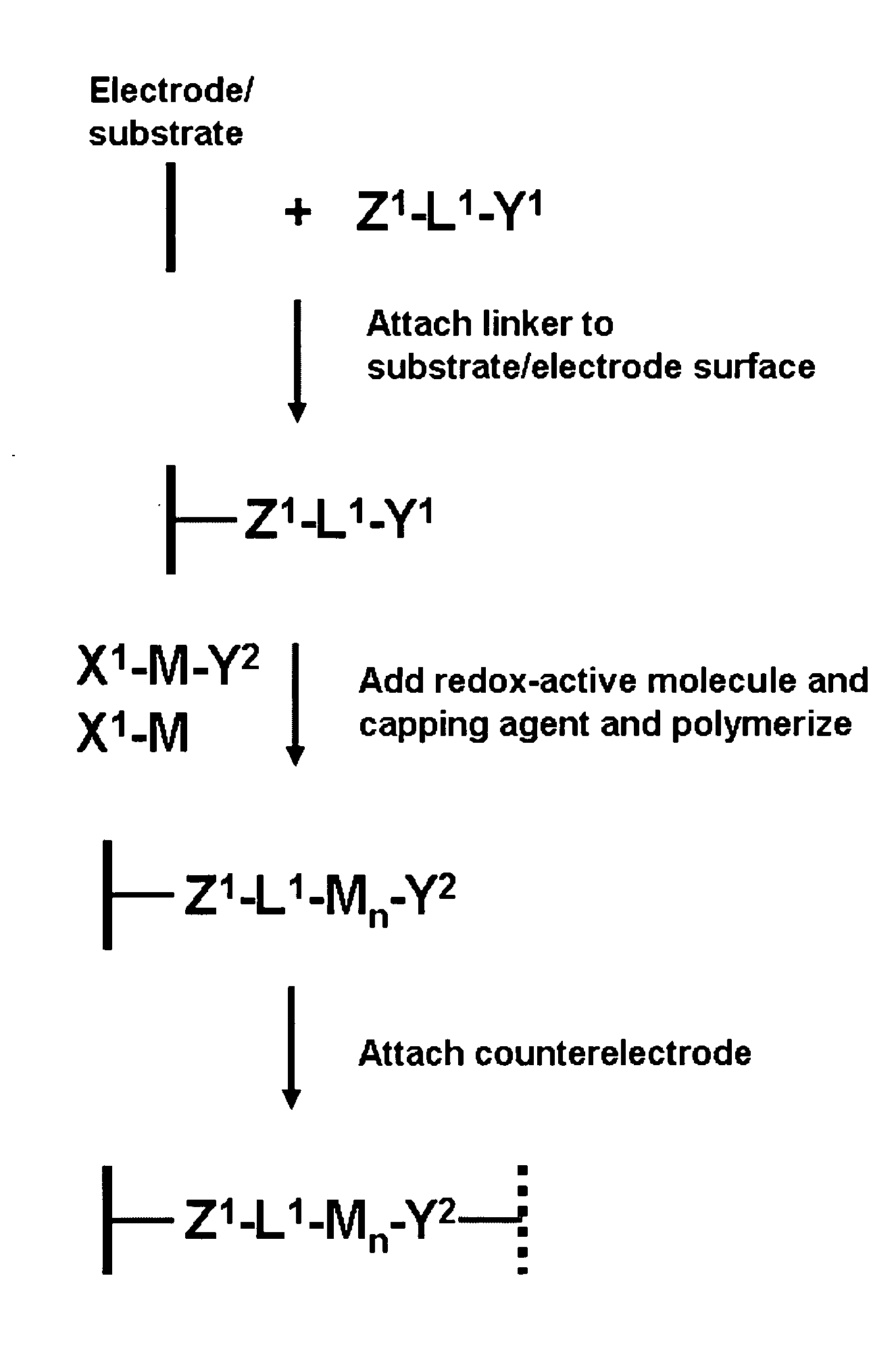
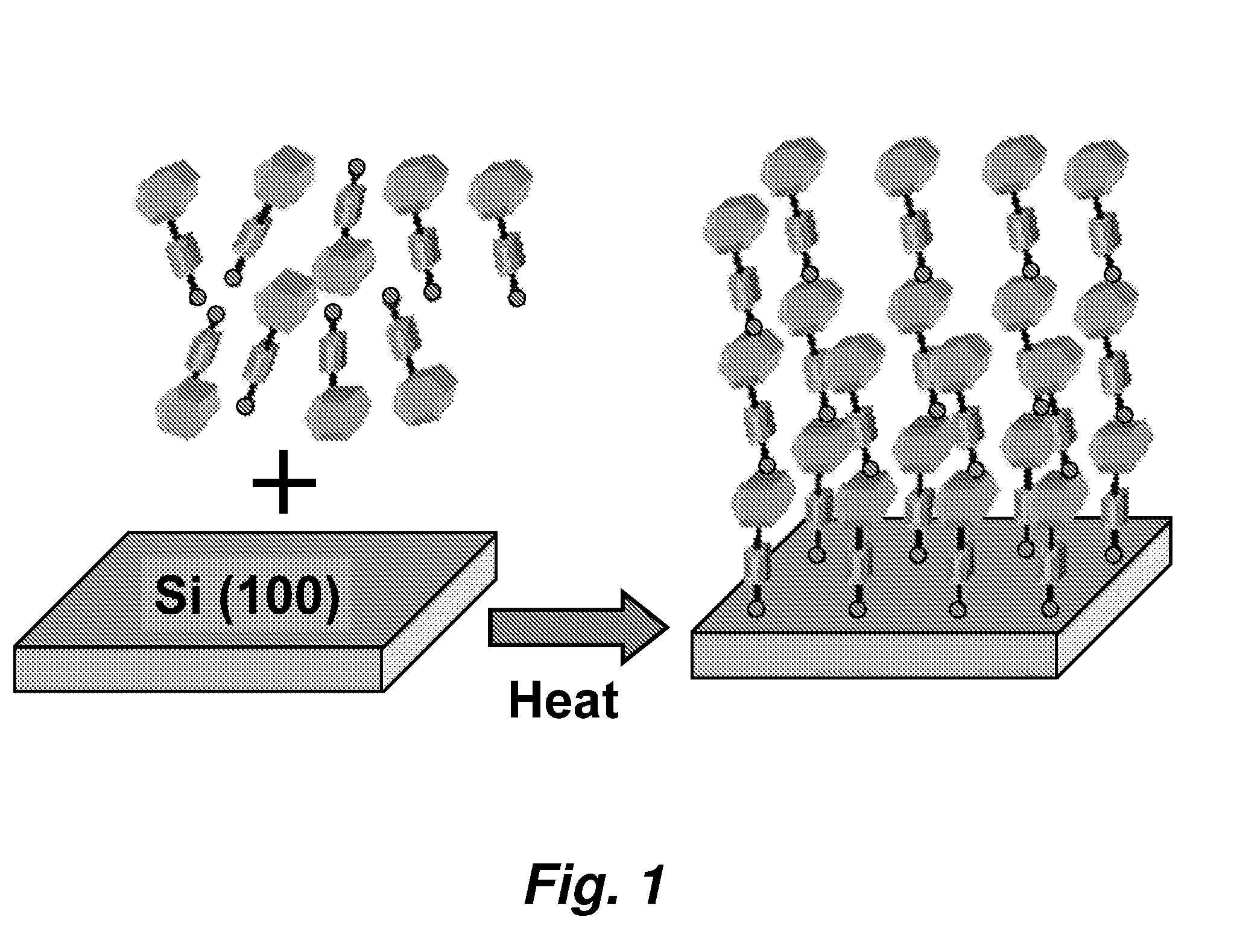
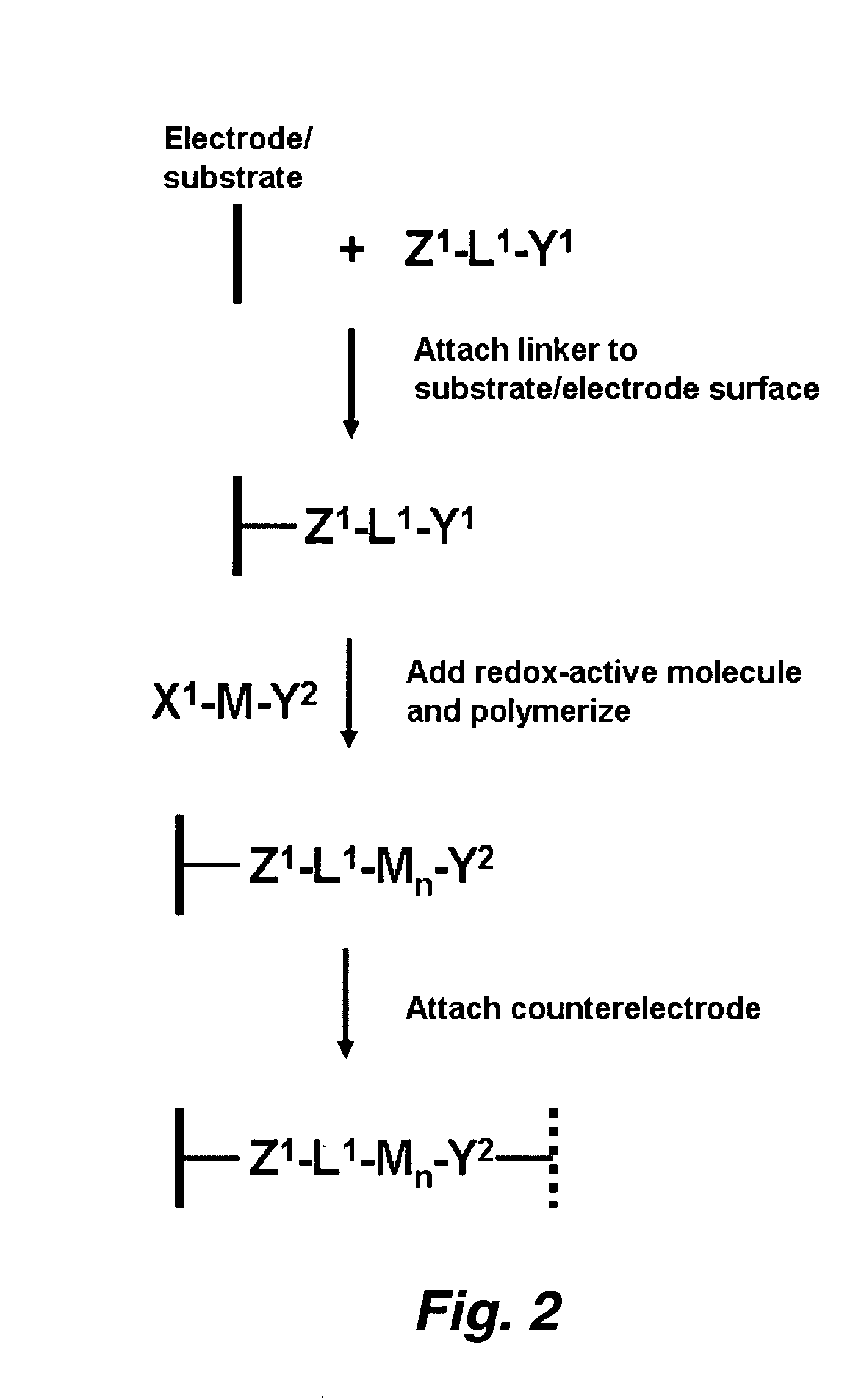
[0138]Porphyrins bearing two (or more) ethynyl groups were made to polymerize under the conditions that we typically employ for forming monolayers on Si (or other) surfaces. The porphyrins were dissolved in a small quantity of organic solvent. Then a small drop of the solvent containing the molecules was deposited on a silicon substrate. The substrate was baked (e.g., at 200° C. to 400° C.) under an inert (argon) atmosphere. The porphyrins formed porphyrin polymers covalently linked to the silicon surface. The polymers were electrochemically robust and exhibit charge-retention times that are comparable (or longer) than those of monolayers of the same molecules.
[0139]FIG. 1 shows the series of molecules that were investigated for polymerization. The polymer can be readily detected electrochemically because the surface coverage is dramatically higher than that exhibited by a monolayer. In partic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com