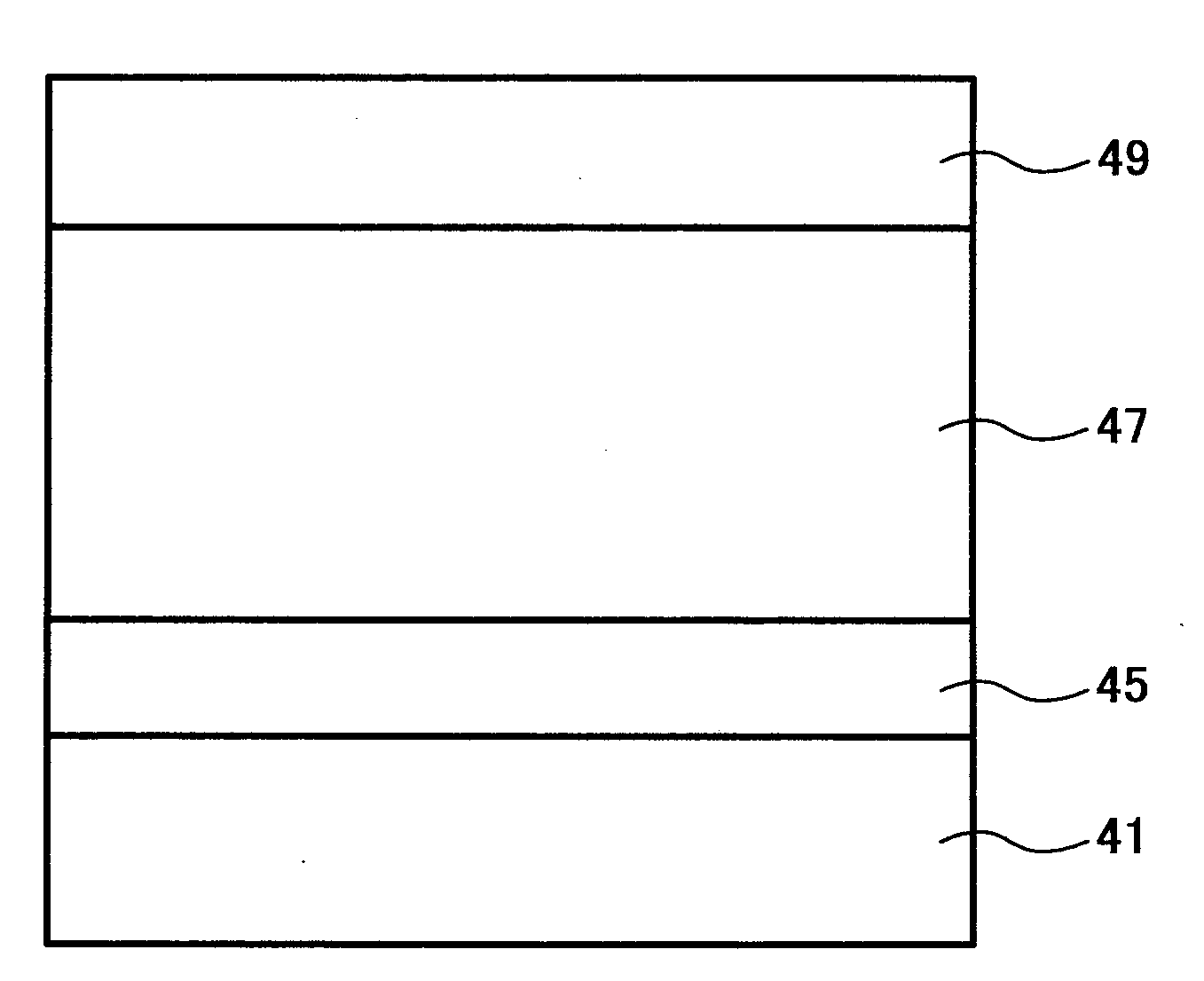
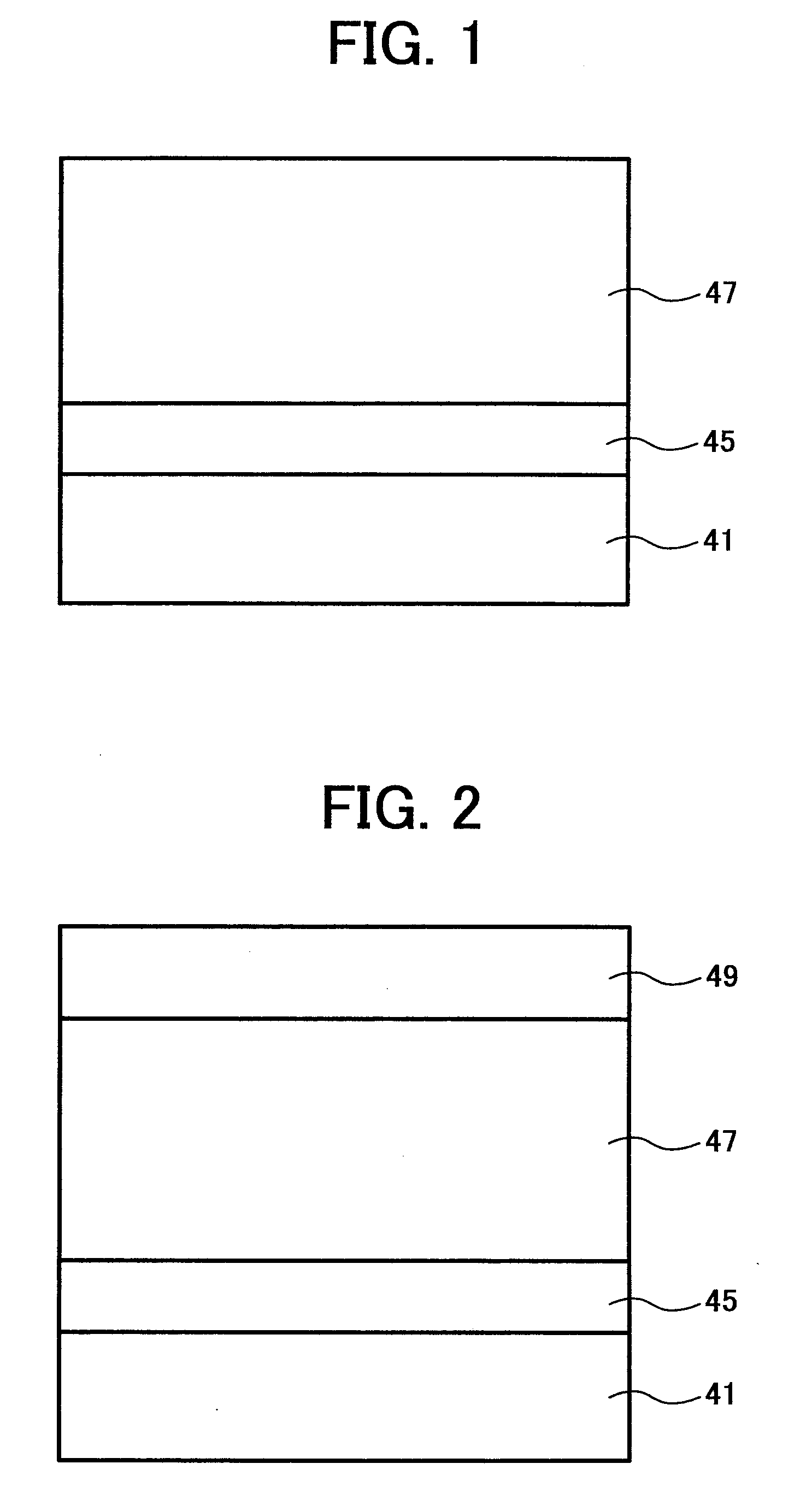
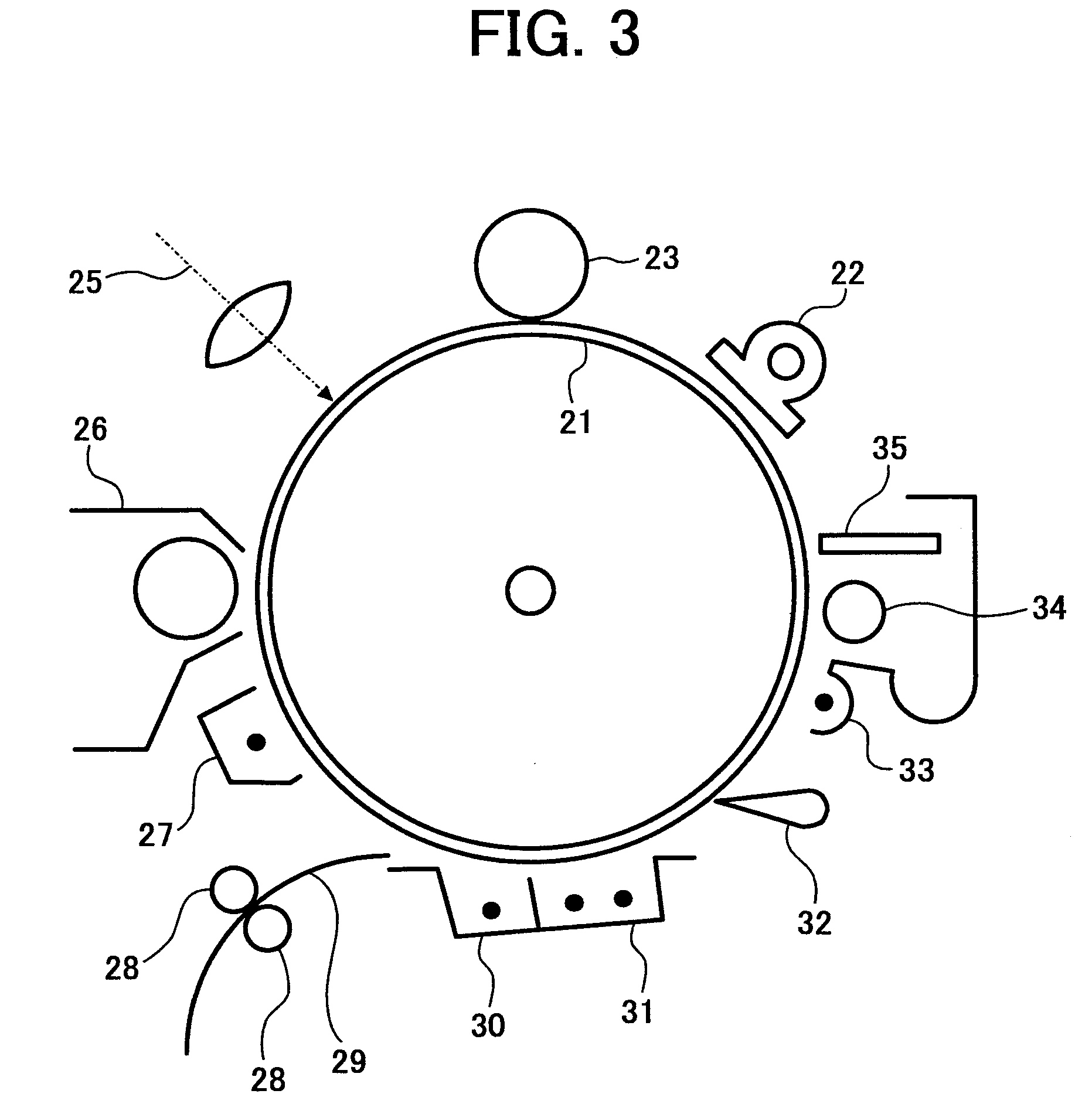
Titanylphthalocyanine crystal and method of producing the titanylphthalocyanine crystal, and electrophotographic photoreceptor, method, apparatus and process cartridge using the titanylphthalocyanine crystal
a technology of titanylphthalocyanine and titanylphthalocyanine, which is applied in the direction of organic dyes, corona discharge, instruments, etc., can solve the problems of fatal defects, background fouling and black spots, and occasional occurrence of point defects, etc., and achieves high crystal stability and small particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 3
[0150]The procedures of preparation for the titanylphthalocyanine crystal in Example 1 were repeated except for changing the crystal conversion solvent from the tetra hydrofuran to dichloromethane to prepare a titanylphthalocyanine crystal. This is a Pigment 3.
examples 1 to 3
and Comparative Examples 1 to 3 had different forms such as forms close to a triangle and forms close to a quadrangle. Therefore, the longest diagonal of the crystal was determined as the major axis.
TABLE 1Average ParticleSize (μm)RemarksExample 10.14The particle sizeswere almost uniform.Example 20.12The particle sizeswere almost uniform.Example 30.15The particle sizeswere almost uniform.Comparative Example 10.16The particle sizeswere almost uniform.Comparative Example 20.25The particle sizesincluded large sizesOf from about 0.3 to0.4 μm.Comparative Example 30.38The particle sizesincluded huge sizesnot less than 0.5 μm.
[0158]A part of the water paste prepared in Example 1 was dried at 80° C. under a reduced pressure (5 mm Hg) for 2 days to prepare a titanylphthalocyanine powder having a low crystallinity.
[0159]X-ray diffraction spectra of the dried powder and the titanylphthalocyanine crystals prepared in Examples 1 to 3 and Comparative Examples 1 to 3 were measured by the following...
example 4
[0175]The following components were dispersed with a commercial beads mill disperser using a PSZ ball having a diameter of 0.5 mm at a rotor revolution speed at 1,500 rpm and the dispersion was stopped when the volume-average particle diameter was less than 0.2 μm to prepare a dispersion liquid. This is a Dispersion Liquid 1.
Titanylphthalocyanine crystal15prepared in Example 1 (Pigment 1)Polyvinylbutyral10(BX-1 from Sekisui Chemical Co., Ltd.)2-butanone280
PUM
| Property | Measurement | Unit |
|---|---|---|
| primary particle diameter | aaaaa | aaaaa |
| Bragg (2θ) angle | aaaaa | aaaaa |
| angles | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com