Compositions controlling pH range of release and/or release rate
a technology of compositions and ph ranges, applied in the direction of drug compositions, biocide, anti-inflammatory agents, etc., can solve the problems of poor soluble compound, poor soluble compound, and difficulty in achieving high-dose doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
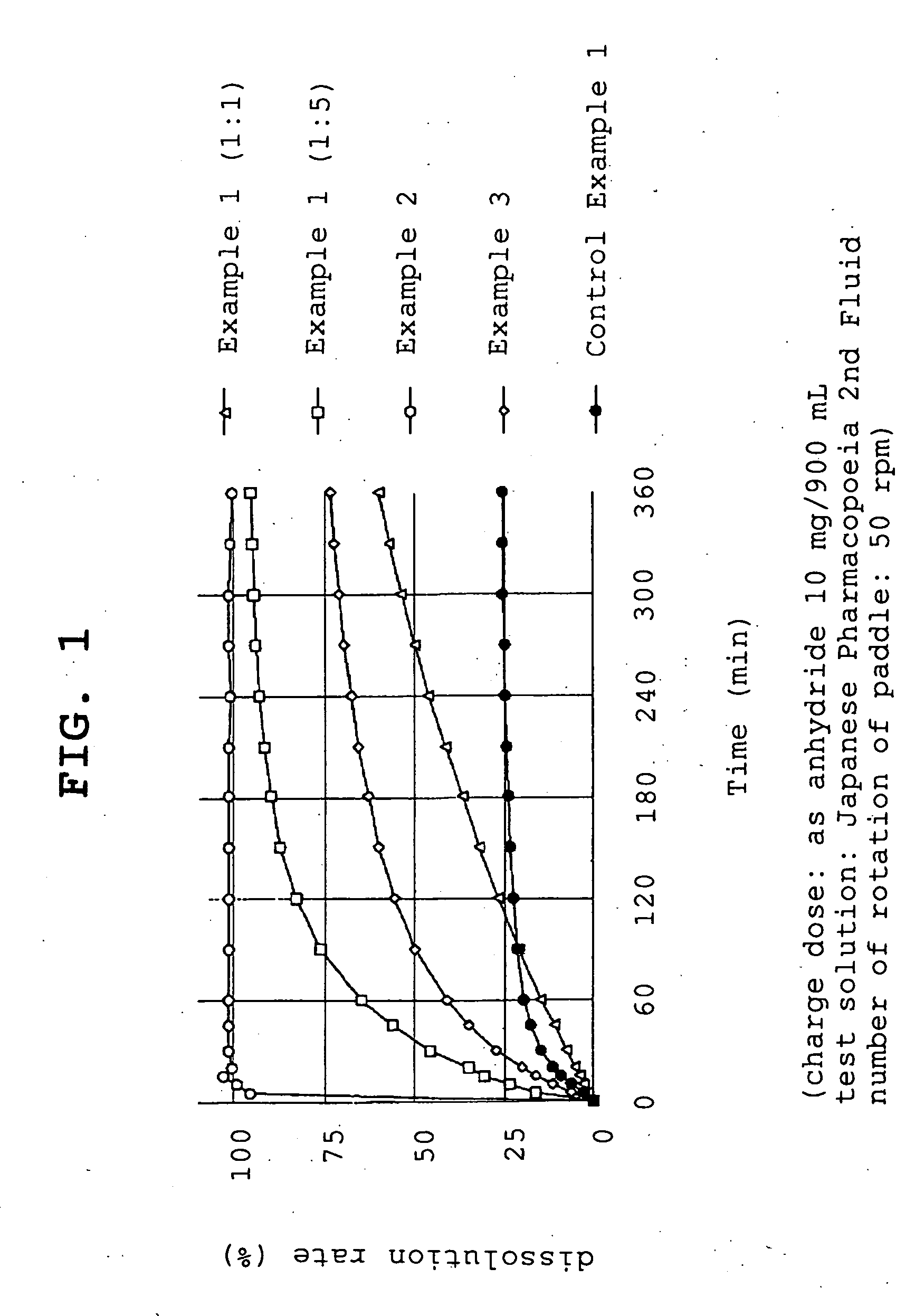
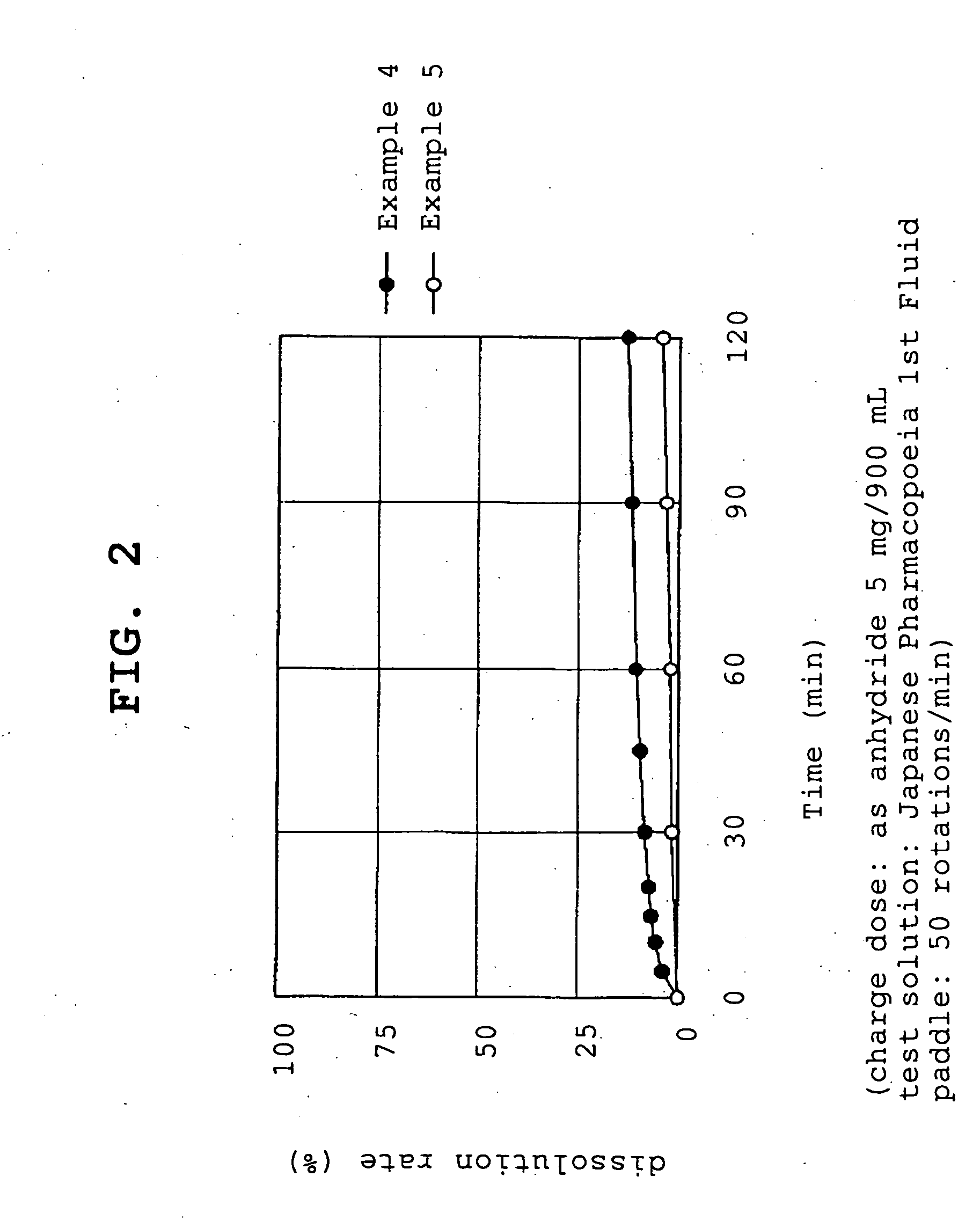
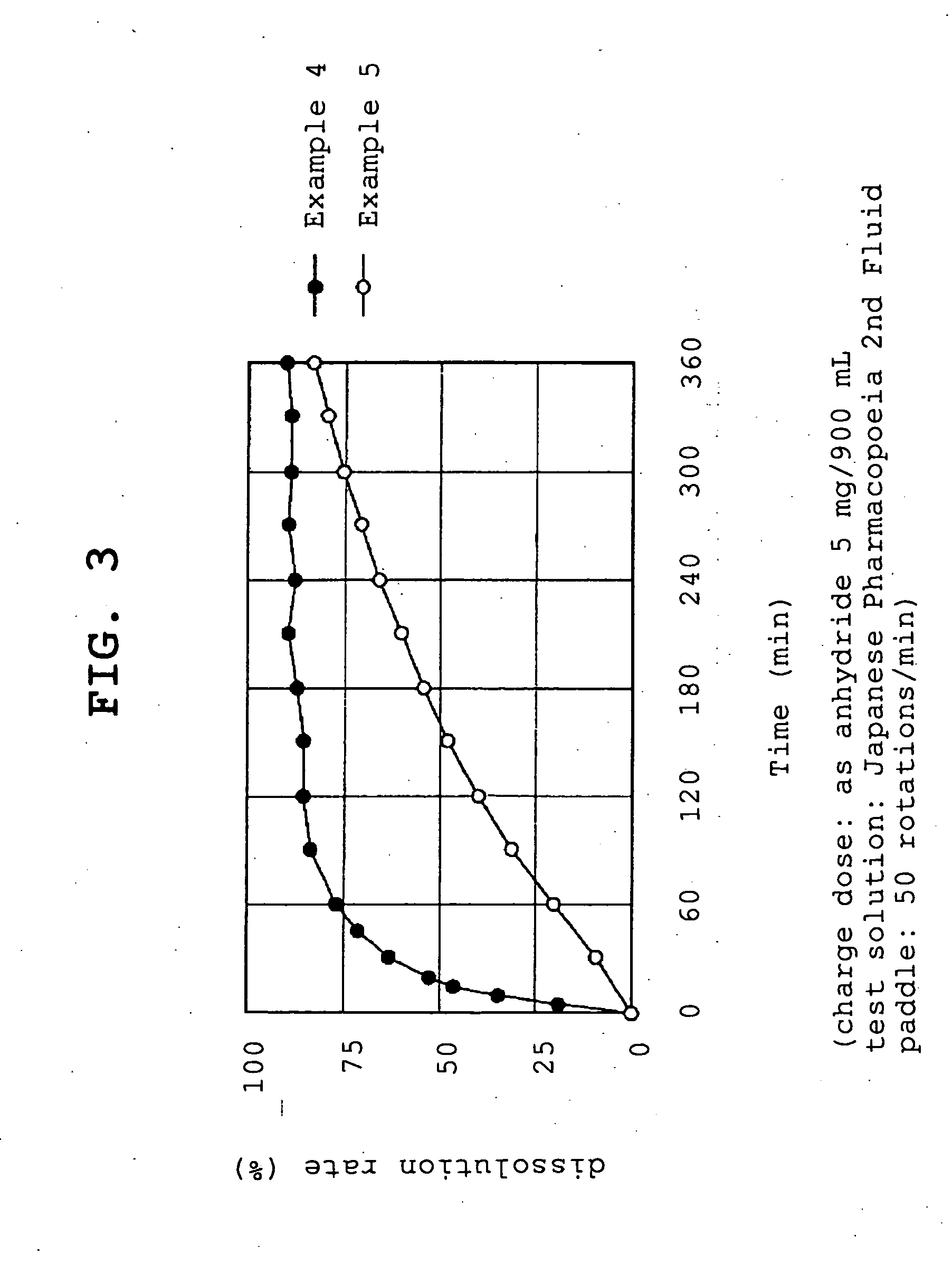
example 1
[0057]Compound A (1.074 g, 1 g by conversion to anhydride) and polyvinylpyrrolidone (1 g or 5 g, product name, Kolidon; manufactured by BASF) were dissolved in ethanol and the organic solvent was evaporated under reduced pressure using a rotary evaporator. The obtained solid was pulverized to give fine granules (a) or (b).
[0058](a)—compound A:polyvinylpyrrolidone=1:1
[0059](b)—compound A:polyvinylpyrrolidone=1:5
example 2
[0060]Compound A (1.074 g, 1 g by conversion to anhydride) and polyoxyethylene hydrogenated castor oil (5 g, product name, HCO-60; manufactured by Nihon Surfactant Kogyo K.K.) were dissolved in ethanol and the mixture was adsorbed to special calcium silicate (1 g, product name, FLORITE RE; manufactured by Eisai Co., Ltd.) to give a powder.
example 3
[0061]Compound A (1.074 g, 1 g by conversion to anhydride) was dissolved in ethanol and the mixture was adsorbed to special calcium silicate (1 g, product name, FLORITE RE; manufactured by Eisai Co., Ltd.) to give a powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com