Compositions for Bacterial Mediated Gene Silencing and Methods of Using the Same
a technology of bacterial mediated gene silencing and compositions, applied in the field of compositions for bacterial mediated gene silencing and methods of using the same, can solve the problems of undeveloped methods, unfavorable side effects, and inability to predict the area of silencing reliably
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Knock Down of Green Fluorescent Protein Using Bacteria Mediated Gene Silencing In Vitro and In Vivo
[0181]In the following experiments, an attenuated strain of Salmonella typhimurium (SL 7207, obtained from BAD Stocker, Stanford University) was used. To prove that the concept is useful as a general approach, we did confirmation experiments also with another attenuated strain of Salmonella typhimurium (VNP 70009, obtained from VION Pharmaceuticals, New Haven) and an invasive and attenuated strain of E. coli (BM 2710, obtained from P. Courvalin, Institut Pasteur, Paris).
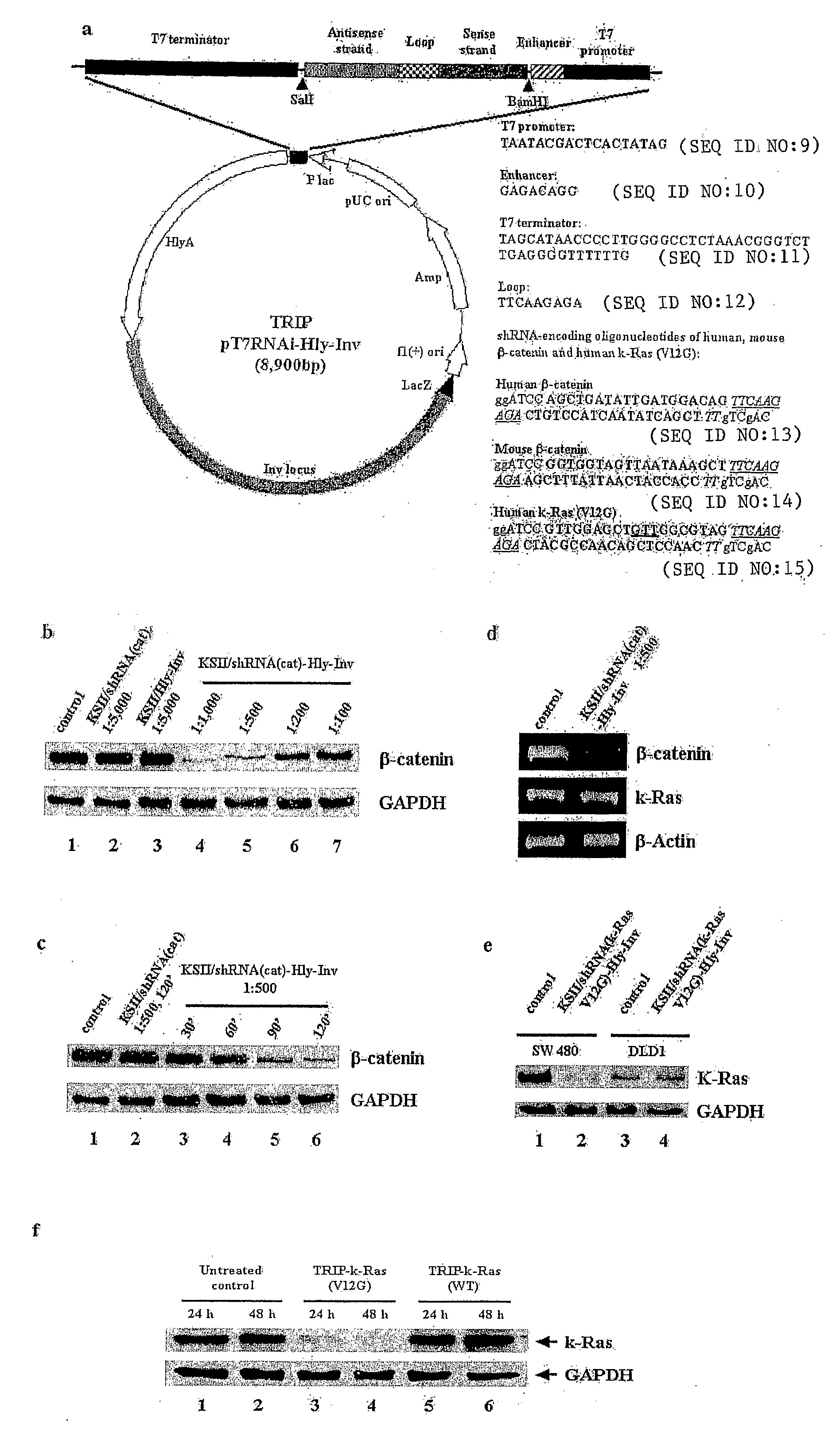
[0182]Silencing plasmids were designed based on a commercially available plasmid (pSilencer, Ambion) to knock down the target genes GFP, β-catenin and oncogenic k-Ras (V12G). These plasmids were transformed into SL 7207 by electroporation and positive clones were verified by growth on selective agar and DNA preparation.
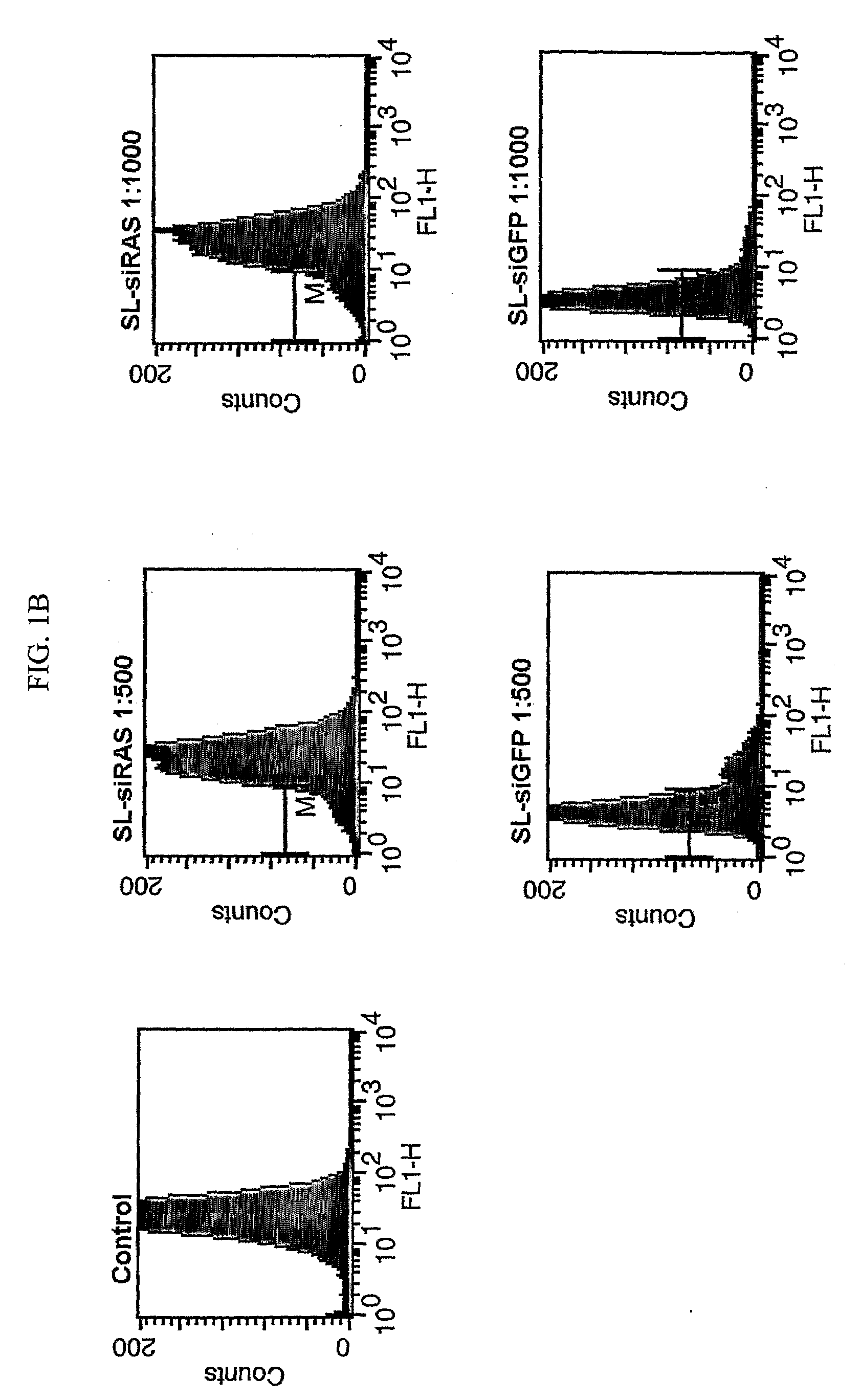
[0183]For in vitro use, knockdown of GFP expression was demonstrated using the stable GFP+ cell line C...
example 2
Knock Down of k-Ras and β-Catenin Using Bacteria Mediated Gene Silencing
[0190]Next, BMGS was applied to knock down a specific disease-related gene. The specific oncogenic point mutation in the k-Ras gene, k-RasV12G, which is present in the human colon carcinoma cell line, SW 480 was targeted.
[0191]After construction of the silencing plasmids and before they were electroporated into the attenuated SL7207, their activity was tested by transfecting them into SW 480 cells using CaP coprecipitation.
[0192]Western blot (FIG. 4A) shows efficient knockdown of k-Ras using the pSilencer-kras (V12G) insert at 36 h and 48 h posttransfection. At later time points, the protein expression recovers, which is due to outgrowth of transfected clones which have a growth disadvantage versus non transfected clones in which the oncogenic k-Ras would still drive replication. When BMGS with SL7207 as a carrier was used to mediate the knockdown, k-Ras levels were decreased at MOI of 1:500 and 1:1000. Using BM...
example 3
In Vivo Bacterial Mediated Gene Silencing
[0197]The method of bacterial mediation of RNAi offers the possibility of selectively targeting more than one gene at a time which might allow for increased efficiency for future applications, e.g. anticancer treatment through interference with multiple oncogenic pathways. To test the feasibility of such an approach, both the mutated k-Ras oncogene and β-catenin were targeted simultaneously. After simultaneous treatment with SL-siRAS and SL-siCAT, knockdown of both genes was observed at the protein level and resulted in further decreased viability and colony formation ability (FIG. 2). These findings demonstrate that the proposed concept of bacterial mediated gene silencing can be successfully used in vitro for different target genes and in different cell lines.
[0198]A mouse model was chosen to test whether this approach can be used to silence target genes in vivo. CgTg5-Nagy mice express high levels of GFP in all tissues. 14 mice were random...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Therapeutic | aaaaa | aaaaa |
| Pharmaceutically acceptable | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com