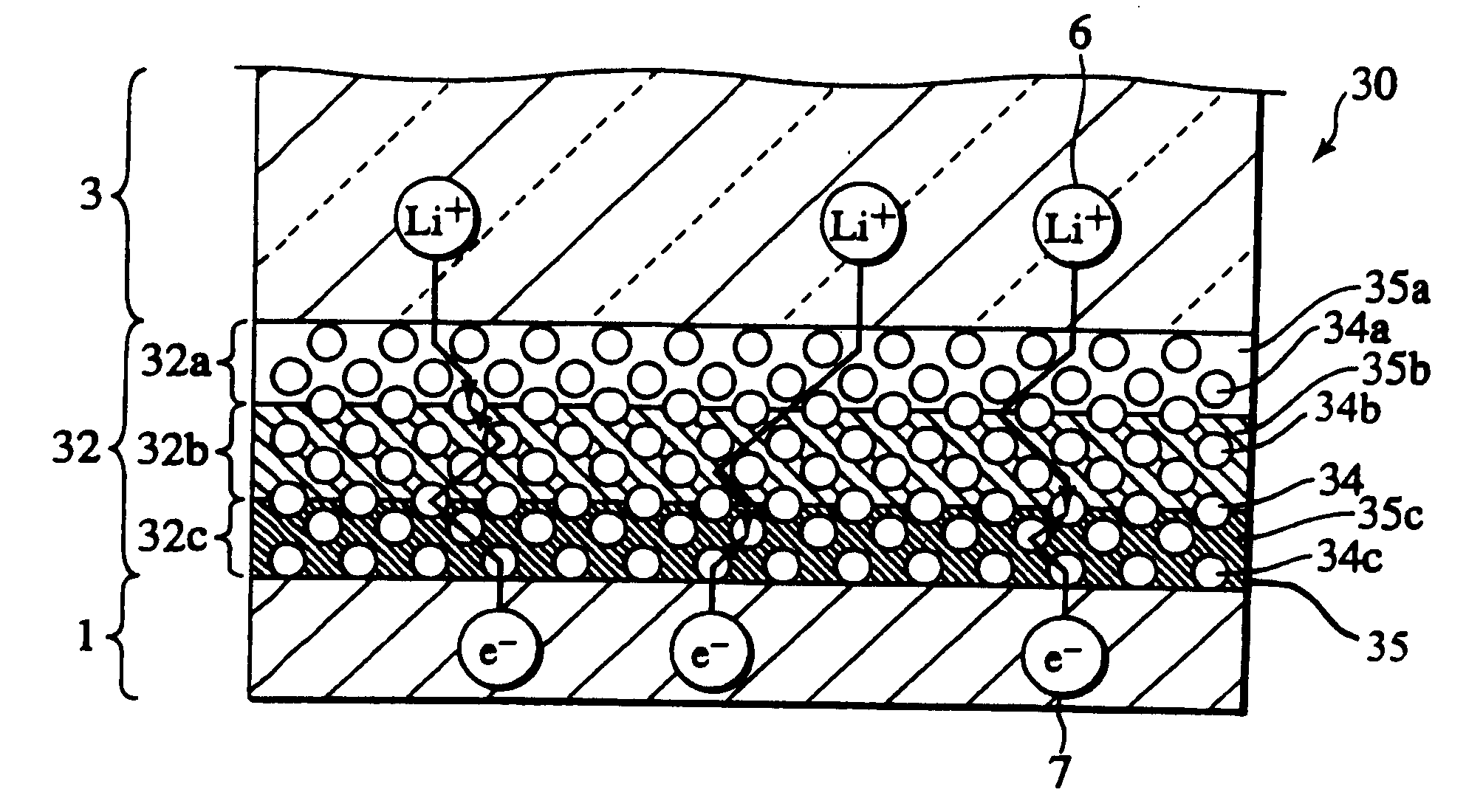
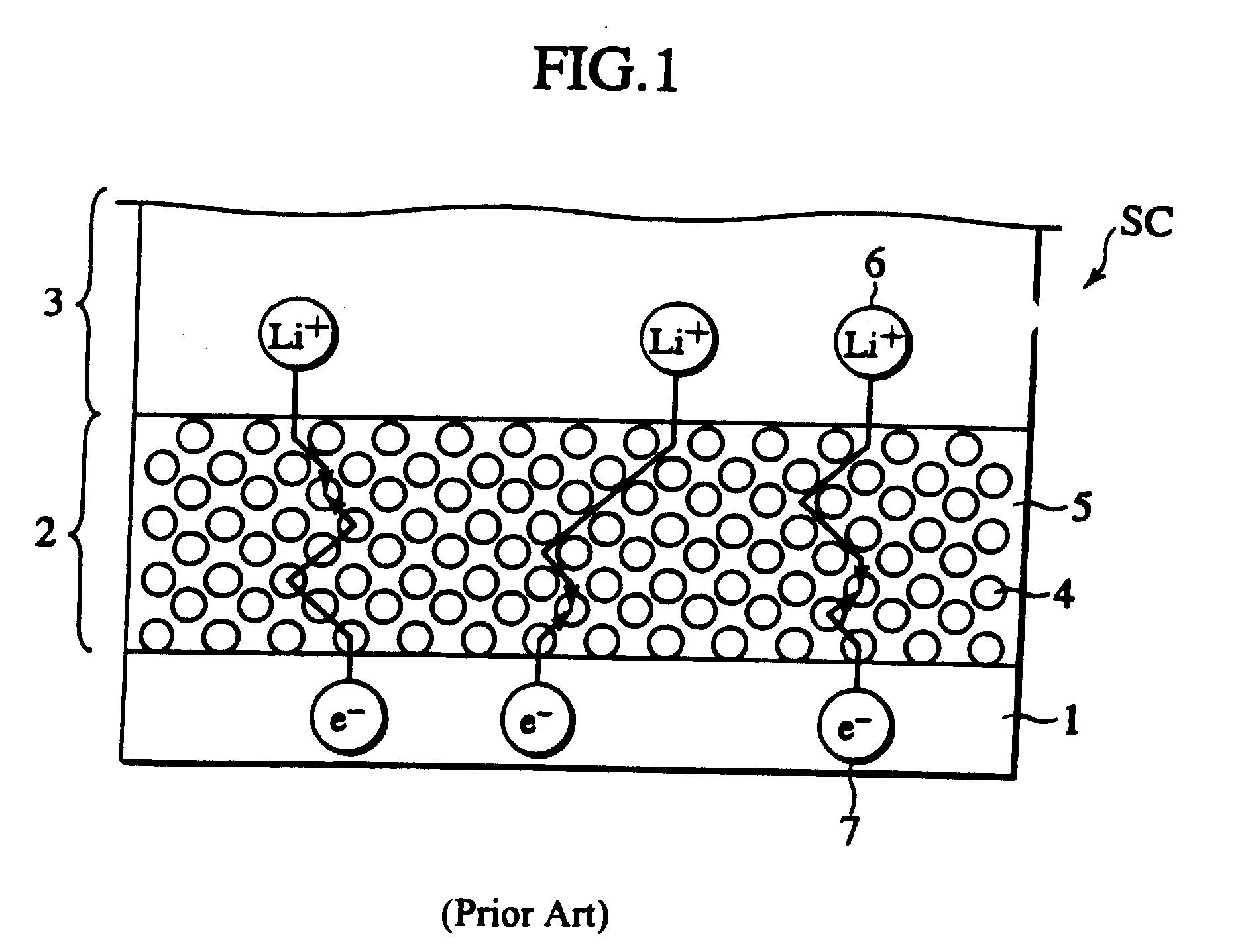
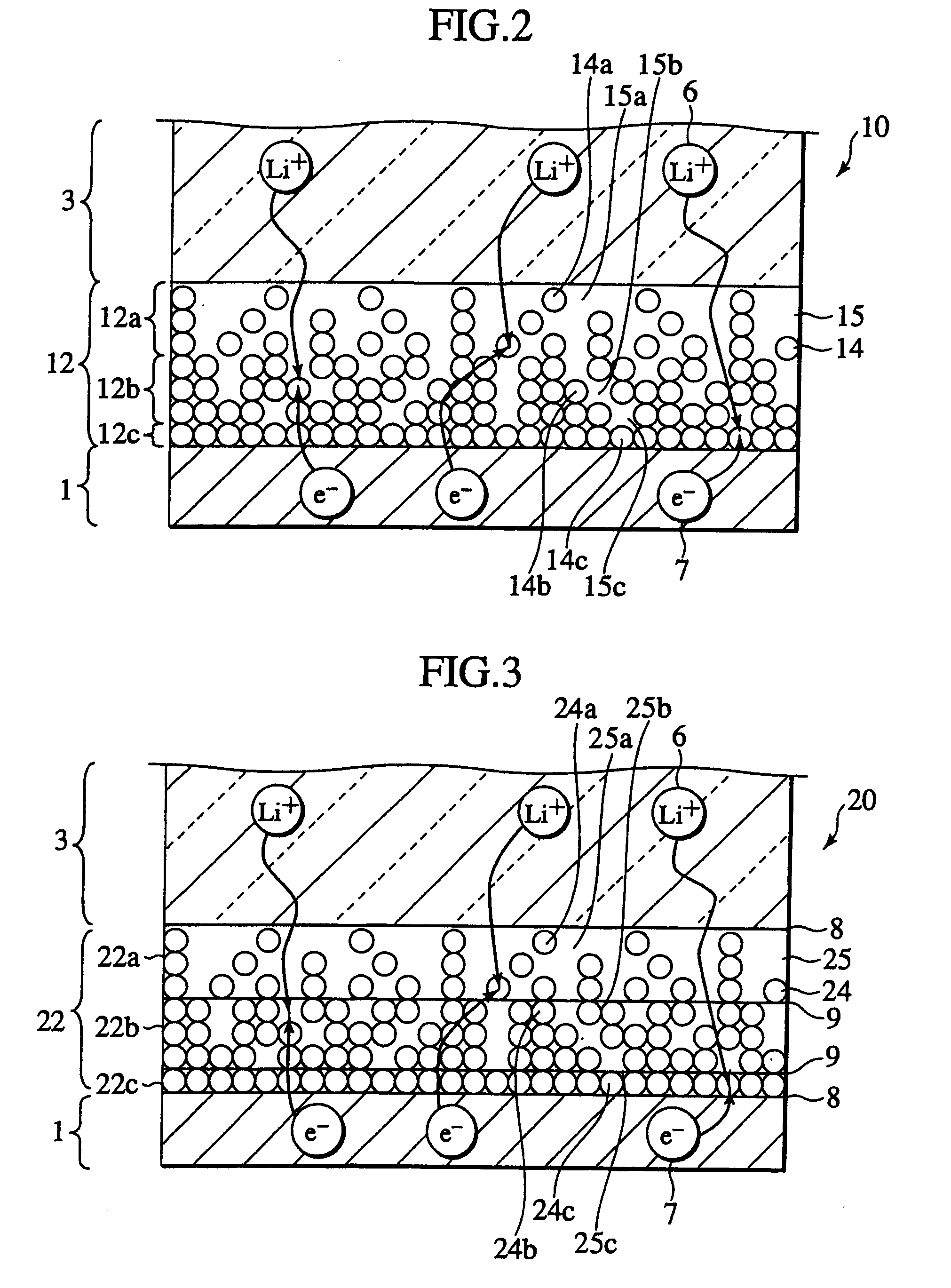
Secondary cell electrode and fabrication method, and secondary cell, complex cell, and vehicle
a technology of secondary cells and electrodes, applied in the field of secondary cells, can solve problems such as the reduction of cell performance such as charge or discharge capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
3.1 Examples of First Embodiment
[0459]The first embodiment is exemplified below.
3.1.1 Example-1
3.1.1a Preparation of positive electrode ink
[0460]A quantity (90 g in weight) of spinel structure LiMn2O4 (particle size: 0.6 μm in average) as a positive electrode active material, a quantity (5 g in weight) of acetylene black as an electrically conductive material, and a quantity (5 g in weight) of polyvinylidene fluoride as a binder were mixed, and as a solvent to this mixture a quantity (300 g in weight) of acetonitrile was admixed, thereby preparing a kind of slurry as a positive electrode ink-1. The positive electrode ink-1 had a viscosity of 3 cP at a temperature of 25° C.
[0461]Next, the positive electrode active material, electrically conductive material, and binder were mixed in the same amounts as those of the positive electrode ink-1, and as a solvent to this mixture a quantity (500 g in weight) of acetonitrile was admixed, thereby preparing another kind of slurry as a positive ...
second embodiment
3.2 Examples of Second Embodiment
[0489]The second embodiment is exemplified below.
3.2.1 Example-2
3.2.1a Preparation of Positive Electrode Ink
[0490]A quantity (90 g in weight) of spinel structure LiMn2O4 (particle size: 0.6 μm in average) as a positive electrode active material, a quantity (5 g in weight) of acetylene black as an electrically conductive material, a quantity (5 g in weight) of polyvinylidene fluoride as a binder, a quantity (40 g in weight) of LiBETl as an electrolyte salt, a quantity (40 g in weight) of macromer of ethylene oxide and propylene oxide as an electrolyte polymer synthesized in accordance with a method described in Japanese Patent Application Laid-Open Publication No. 2002-110239, and a quantity (0.1 mass % of the electrolyte polymer) of benzyldimethyl-ketal as a photochemical polymerization initiator were mixed, and as a solvent to this mixture a quantity (820 g in weight) of acetonitrile was admixed, thereby preparing a kind of slurry as a positive elec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com