Adeno-associated virus vectors
a technology of plasmid-based vectors and vectors, which is applied in the field of adeno-associated virus vectors, can solve the problems of modulating the efficiency of transduction and persistence, little is known, etc., and achieves the effects of increasing episomal stability, abundance and stability, and increasing the stability of plasmid-based vectors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
[0117]Construction of rAAV Shuttle Vector.
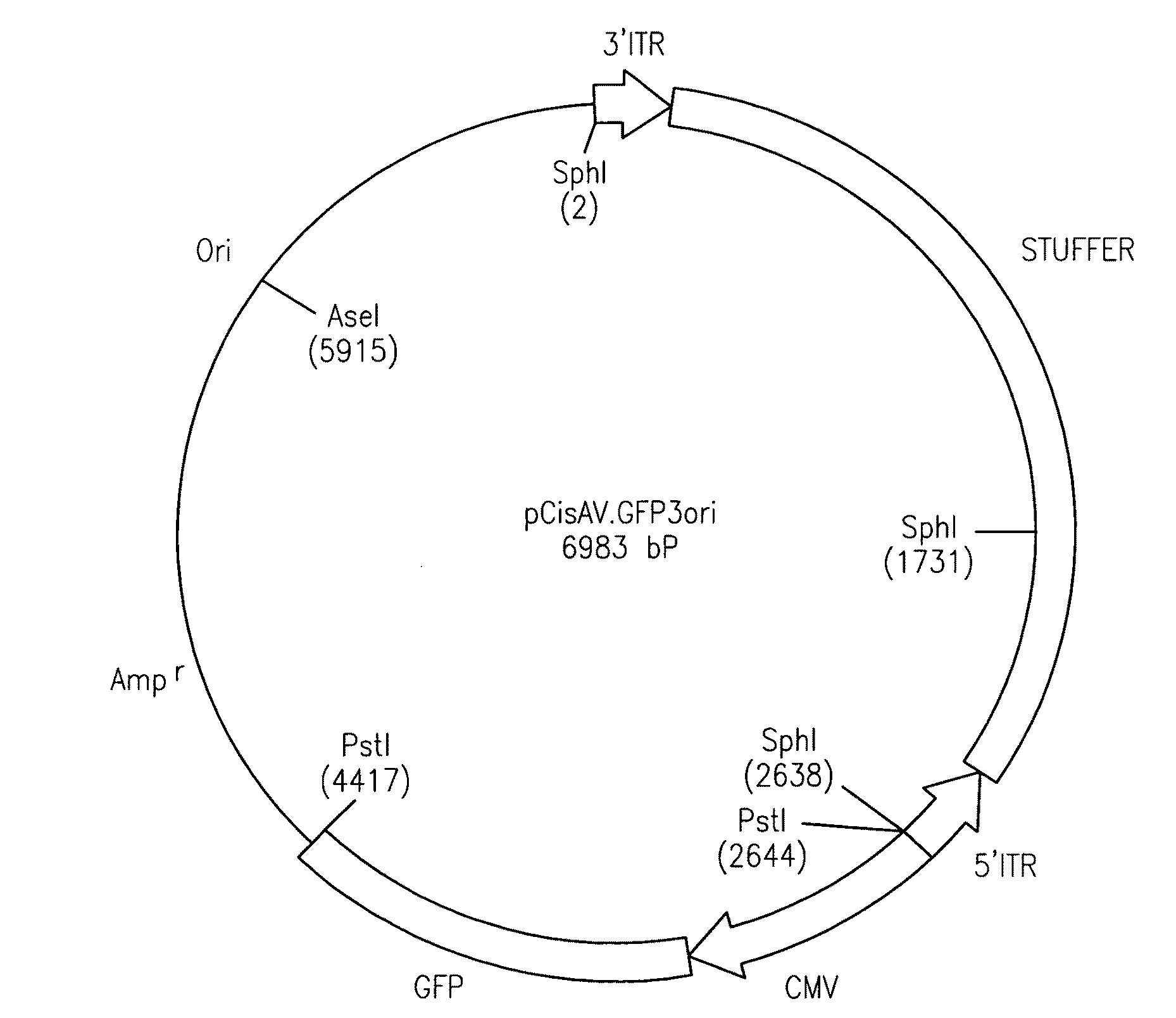
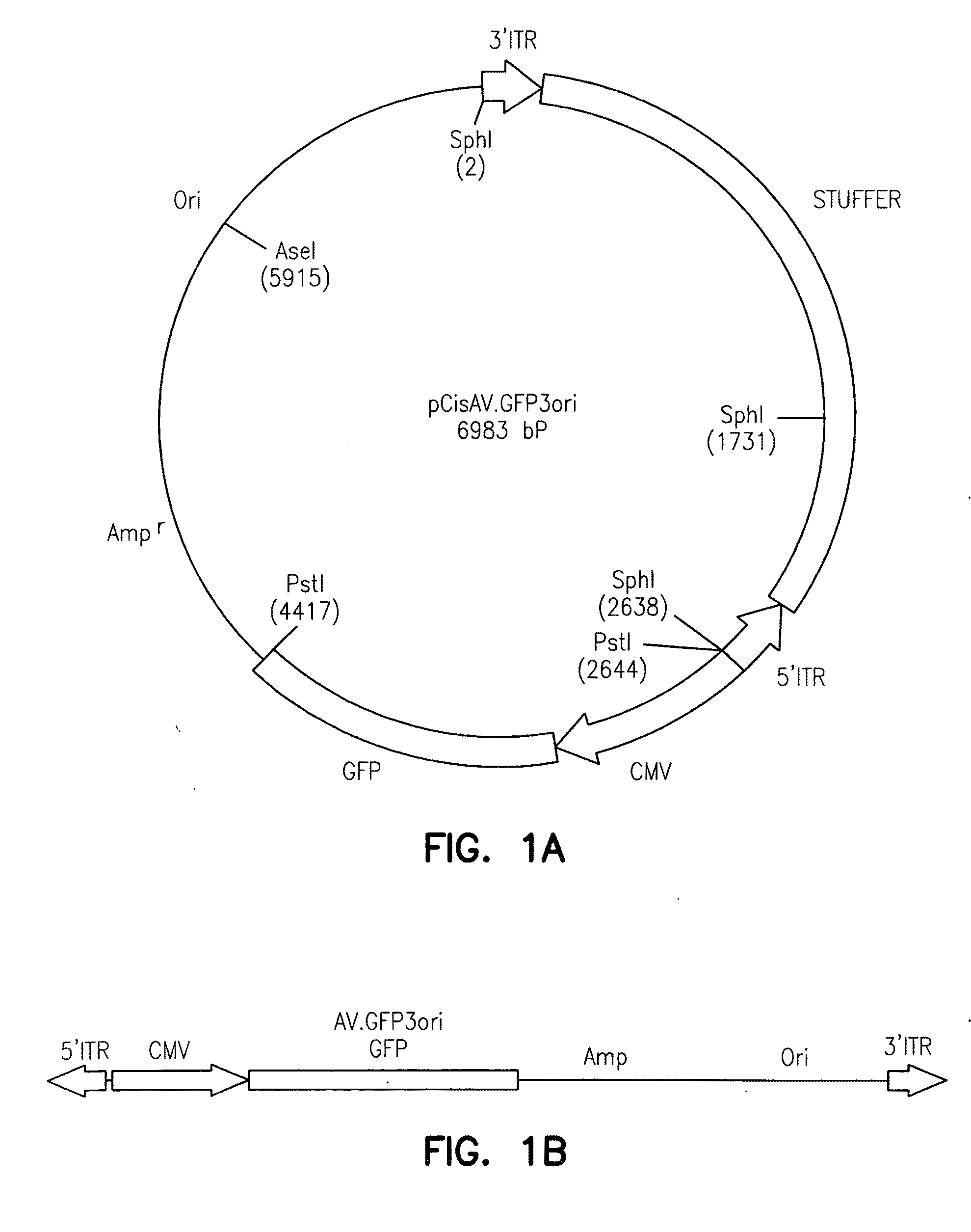
[0118]A recombinant AAV shuttle vector (AV.GFP3ori) which contained a GFP transgene cassette, bacterial ampicillin resistance gene, and bacterial origin of replication, was generated from a cis-acting plasmid (pCisAV.GFP3ori). Expression of the GFP gene was directed by the CMV promoter / enhancer and SV40 poly-adenylation sequences. pCisAV.GFP3ori was constructed with pSub201 derived ITR elements (Samulski et al., 1987) and the intactness of ITR sequences was confirmed by restriction analysis with SmaI and PvuII, and by sequencing. Recombinant AAV stocks were generated by co-transfection of pCisAV.GFP3ori and pRep / Cap together with co-infection of recombinant Ad.CMVlacZ in 293 cells (Duan et al., 1997). Following transfection of forty 150 mm plates, cells were collected at 72 hours by centrifugation and resuspended in 12 ml of buffer (10 mM Tris pH 8.0). Virus was released from cells by three cycles of freeze / thawing and p...
example 2
Methods
[0140]Production of rAAV Shuttle Vector.
[0141]The cis-acting plasmid (pCisAV.GFP3ori) used for rAAV production was generated by subcloning the Bsp1201 / Not I fragment (743 bp) of the GFP transgene from pEGFP-1 (Clontech) between the CMV enhancer / promoter and SV40polyA by blunt-end ligation. A 2.5 kb cassette containing beta-lactamase and bacterial replication origin from pUC19 was blunt ligated down-stream of GFP reporter cassette. The ITR elements were derived from pSub201.2 The entire plasmid contains a 4.7 kb AAV component flanked by a 2 kb stuffer sequence. The integrity of ITR sequences was confirmed by restriction analysis with SmaI and PvuII, and by direct sequencing using a modified di-deoxy procedure which allowed for complete sequence through both 5′ and 3′ ITRs. Recombinant AAV stocks were generated by co-transfection of pCisAV.GFP3ori and pRep / Cap together with co-infection of recombinant Ad.CMVlacZ in 293 cells. The rAV.GFP3ori virus was subsequently purified thro...
example 3
Evidence for Increased Episomal Persistence of AAV Circular Intermediates in a Model for In Utero Plasmid-Based Gene Therapy
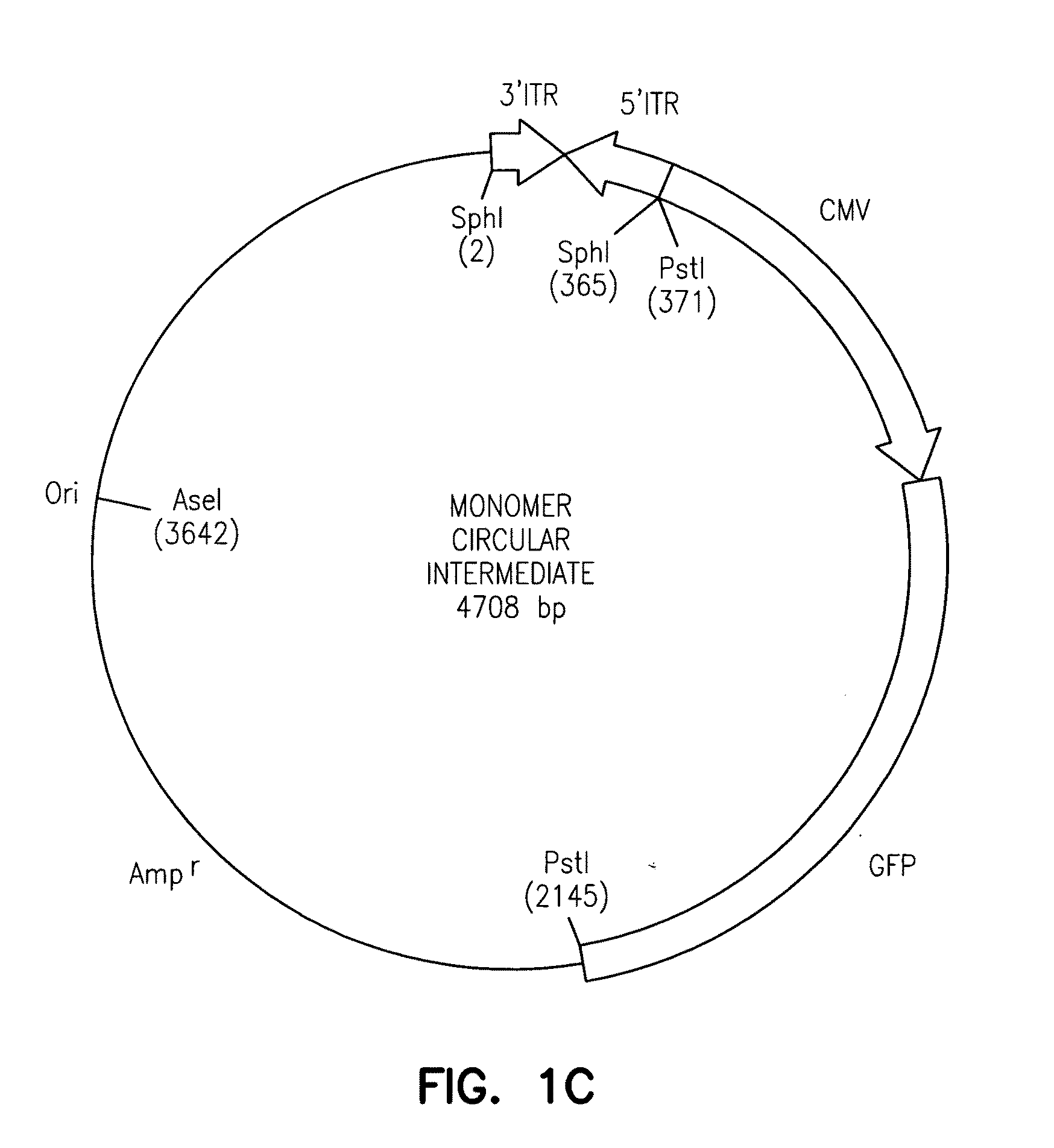
[0159]Persistence of AAV circular intermediates were assessed by injection of plasmid DNA directly into the pronucleus of fertilized Xenopus oocytes. Twenty-five ng of the p81 isolate of AAV circular intermediates was injected at the single cell stage of fertilized Xenopus oocytes. This plasmid was compared to the proviral plasmid pCisAV.GFP3ori, which contains two ITRs separated by stuffer sequence in an alternative confirmation to ITRs in p81. FIG. 13 depicts the persistence of GFP plasmids as assessed by direct fluorescence of GFP. At this state of tadpole development, the fertilized oocyte has expanded from a single cell to approximately 106 cells.
[0160]These studies confirm that AAV circular intermediates (p81) confer a higher level of stability in development Xenopus oocytes than plasmids containing similar transcriptional elements and ITR sequences in an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com