Multilayer material based on active lithium, method of preparation and applications in electrochemical generators
a multi-layer material and active lithium technology, applied in the field of multi-layer materials based on active lithium, can solve the problems of increasing the cost of industrial equipment, increasing the complexity of the industrial level, and requiring more complicated and costly devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0127]A half-cell is prepared consisting of a current collector, a cathode material and a solid polymer electrolyte SPE.
[0128]The cathode material consists of LiV3O8, a polyether binder, LiTFSI and a high specific surface area carbon. The cathode has a thickness of 45 μm.
[0129]The electrolyte SPE consists of a solution of LiTFSi in a polyether type of polymer, and its thickness is between 20 and 30 μm.
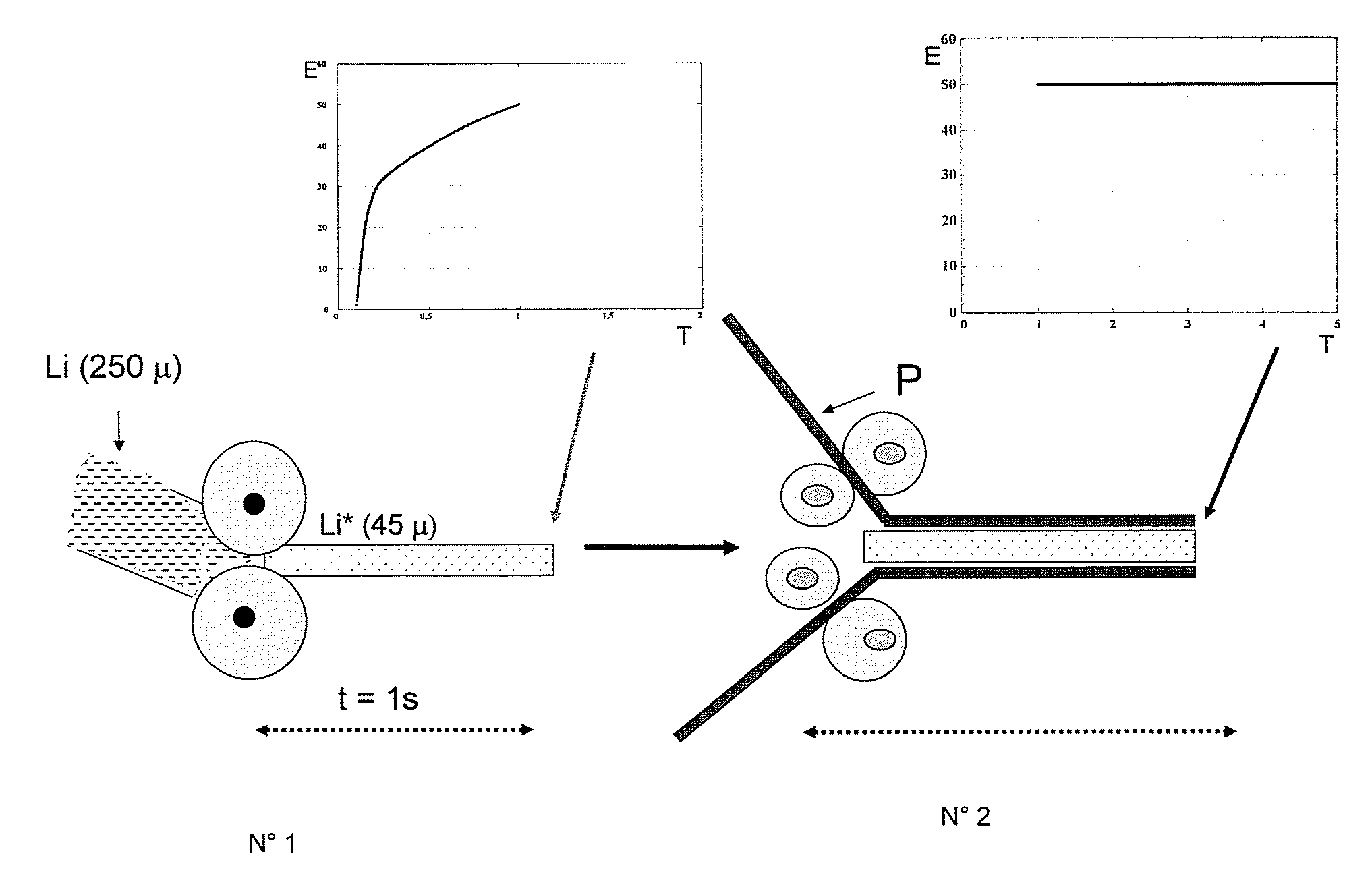
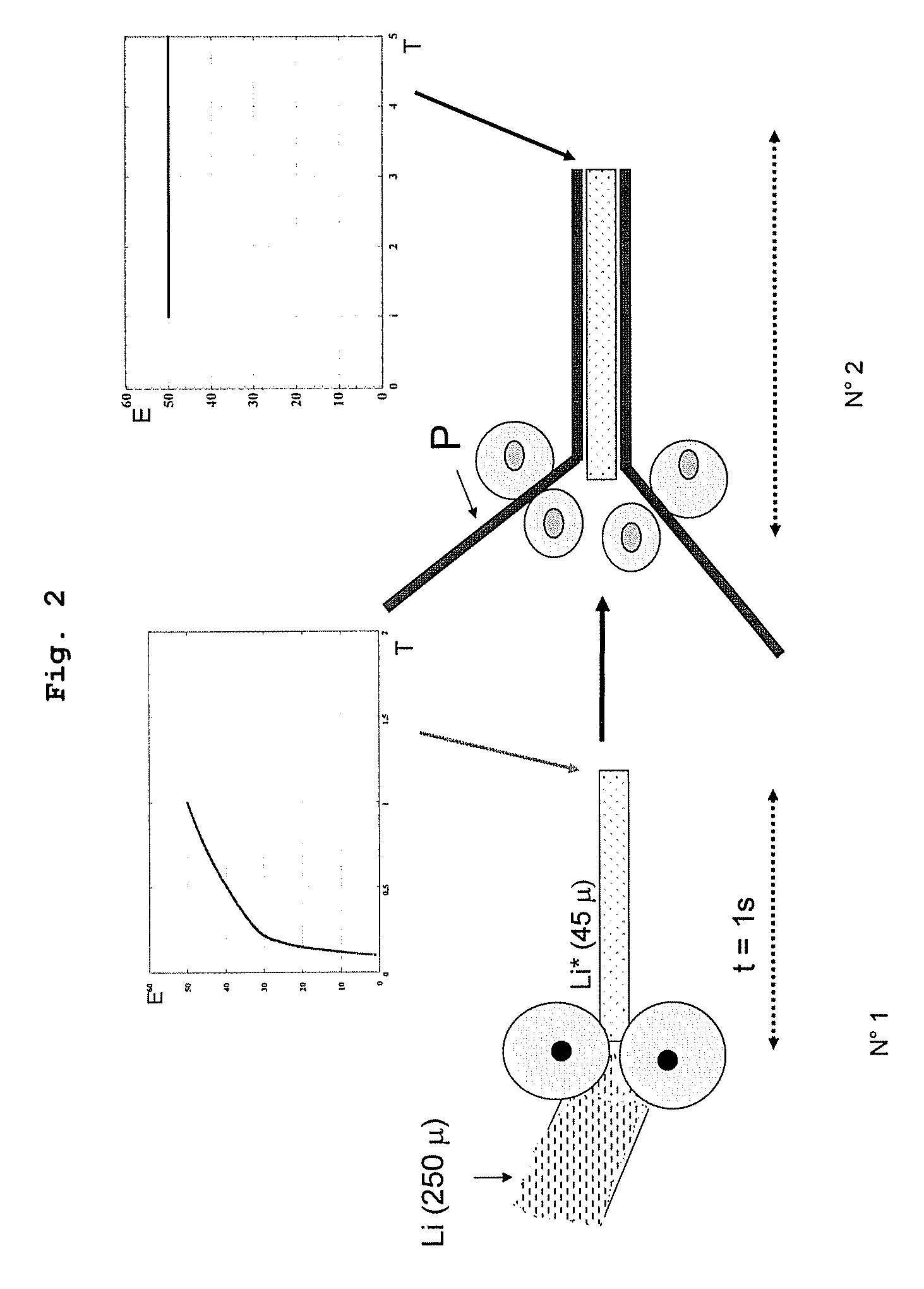
[0130]A film of metal lithium having a thickness of 250 μm is obtained by extrusion, and then manually laminated with a jeweler's roll mill for 2 seconds. A lithium film having a thickness of 55 μm is thus obtained, with a passivation film on its furnace of which the thickness is 25 Å.
[0131]This film is extremely sticky and adheres to the assembly on the LiV3O8 / SPE cell. The half-cell has an impedance of 6Ω, which is much lower, hence more advantageous, than the impedance of 12Ω of a cell containing a standard lithium film at the same measurement temperature of 60° C.
example 2
[0132]A film of methyl lithium having a thickness of 250 μm is obtained by extrusion. It is then laminated with a jeweler's roll mill, at ambient temperature for 2 seconds. A film of active lithium, having a thickness of 55 μm, is thus obtained, and it has a passivation layer having a thickness of 45 Å. This film was evaluated on the same day in an XPS analyzer. The thickness measured for the Li2O layer is 255 Å.
[0133]A film of active lithium which remained for one week in an anhydrous chamber has a Li2O layer having a thickness of 250 Å and a Li2CO3 layer having a thickness of 125 Å.
[0134]These values should be compared with those of the commercial lithium from FMC, in which the Li2O layer has a thickness of 400 Å and the Li2CO3 has a thickness of 150 μm.
example 3
[0135]A half-current collector / cathode material / SPE cell is prepared by the method of example 1.
[0136]A LIPON layer is deposited by sputtering on the SPE face of the half-cell, from a Li3PO4 target. It has a thickness of 900 nm and an adhesiveness, measured by ASTM method number D3359, of 5 / 5.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com