Human cytomegalovirus neutralising antibodies and use thereof
a technology of cytomegalovirus and neutralizing antibodies, which is applied in the field of human cytomegalovirus neutralising antibodies, can solve the problems of modest neutralizing potency of antibodies isolated so far, and hcmv is also known to cause pathology, and achieves high potency in neutralizing hcmv
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cloning of B Cells and Screening for hCMV Neutralizing Activity
[0192]Donors with high hCMV neutralizing antibody titres in the serum were identified. Memory B cells were isolated and immortalised using EBV and CpG as described in reference 36. Briefly, memory B cells were isolated by negative selection using CD22 beads, followed by removal of IgM+, IgD+ IgA+ B cells using specific antibodies and cell sorting. The sorted cells (IgG+) were immortalized with EBV in the presence of CpG 2006 and irradiated allogeneic mononuclear cells. Replicate cultures each containing 50 memory B cells were set up in twenty 96 well U bottom plates. After two weeks the culture supernatants were collected and tested for their capacity to neutralize hCMV infection of either fibroblasts or epithelial cells in separate assays. B cell clones were isolated from positive polyclonal cultures as described in reference 36. IgG concentrations in the supernatant of selected clones were determined using an IgG-speci...
example 2
Identification of the Target Antigens Recognized by the Monoclonal Antibodies
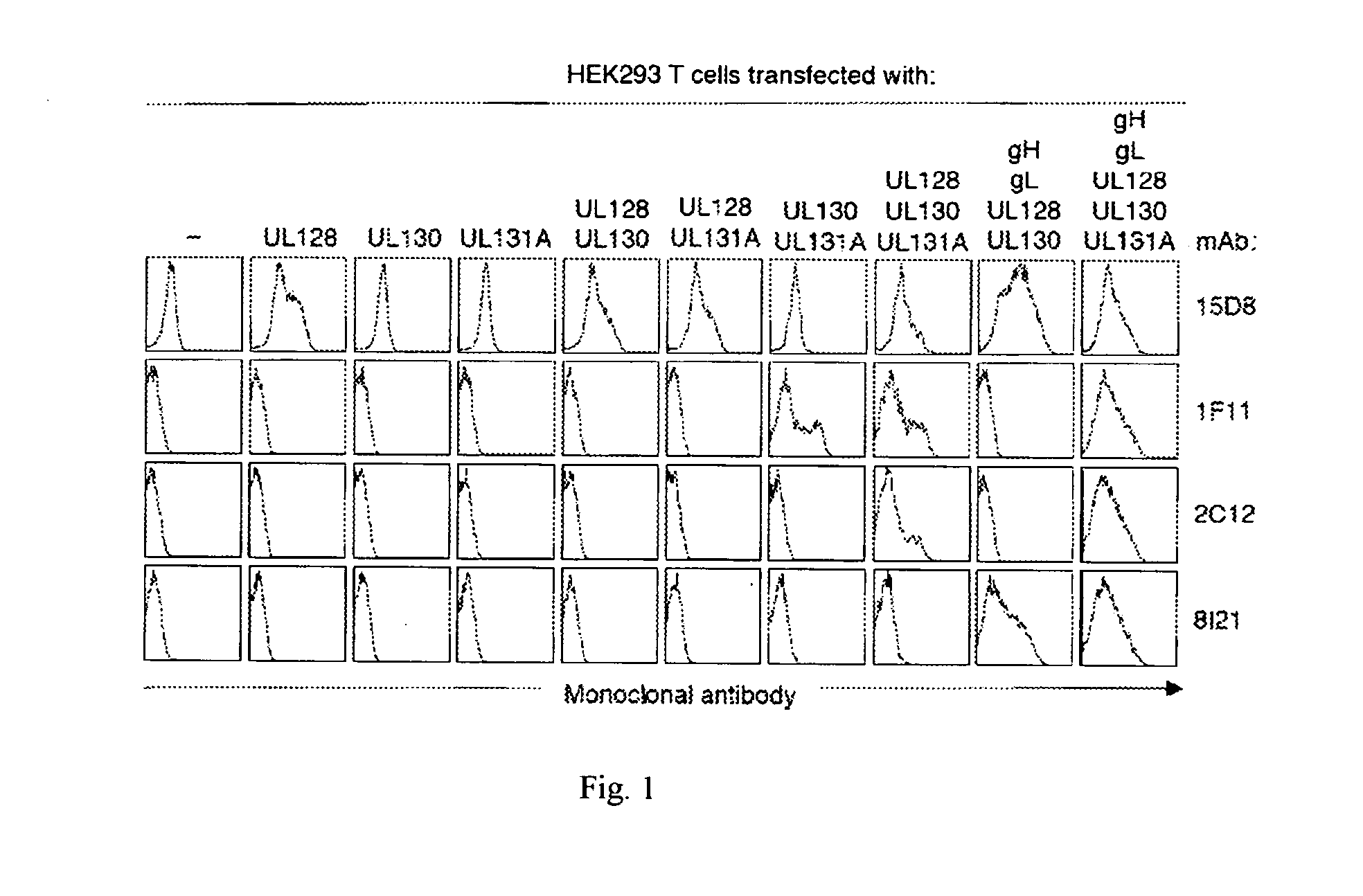
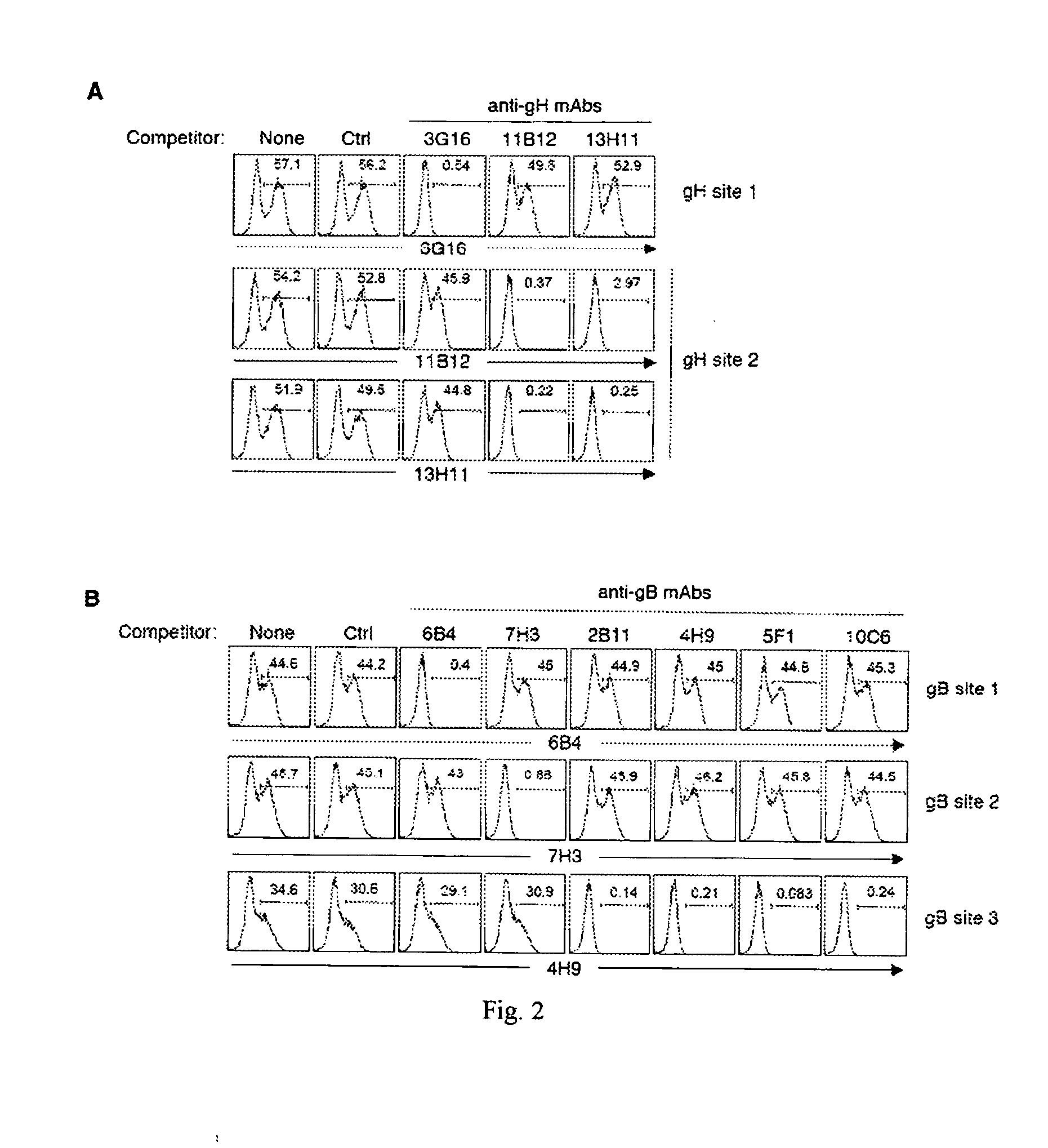
[0197]To map the specificity of the hCMV neutralizing antibodies, HEK293T cells were transfected with one or more vectors encoding full length hCMV proteins UL128, UL130, UL131A, gH, gL, gB, gM, and gN. After 36h, cells were fixed, permeabilized and stained with the human monoclonal antibodies followed by goat anti-human IgG. FIG. 1 shows the binding of representative antibodies to HEK293T cells expressing one or more hCMV proteins. Table 6 shows the staining pattern of all the different antibodies to hCMV gene-transfected HEK293T cells. With the exception of antibody 15D8, that stained UL128-transfected cells, all the other Group 2 antibodies did not stain single gene transfectants, suggesting that they may recognize epitopes that require co-expression of more than one gene product. Indeed, five antibodies (4N10, 10F7, 10P3, 4I22 and 8L13) stained cells co-expressing UL130 and UL131A, six antibodies (2C12,...
example 3
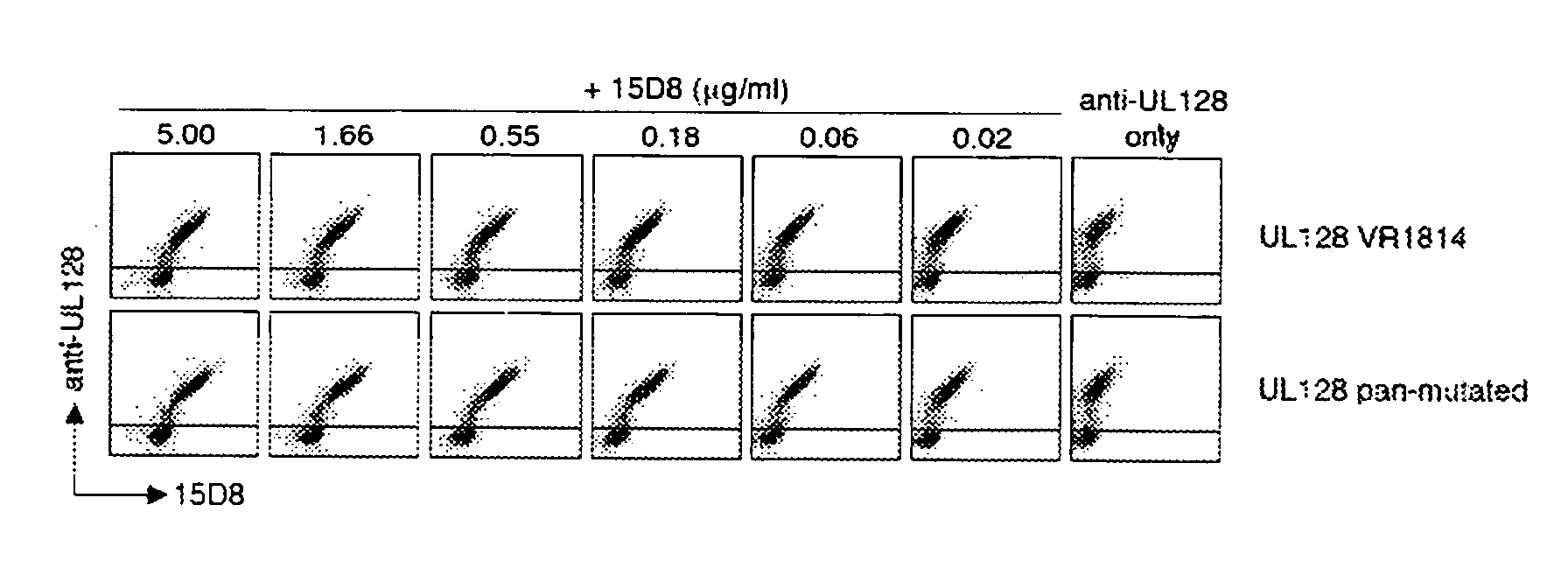
Breadth of Neutralizing Activity of Antibody 15D8
[0220]UL128 is the most conserved gene of the UL132-128 locus. However, sequences derived from several clinical isolates revealed the existence of 10 variants with one or more mutations when compared to the VR1814 sequence. We therefore investigated whether the binding of the UL128-specific antibody 15D8 would be affected by any of these mutations. To this aim, published amino acid sequences of variants of UL128 from clinical isolates (VR4603-M, VR4836-M, VR5001-M, VR4254-M, VR4969-M, VR4313-M, VR4116-M, VR5235-T, VR5055-T, VR4168-A, VR1814-PCR) and laboratory strains (Towne, TB40 / E, AD169, Merlin and Toledo) were aligned, and a gene was synthesized encoding a protein that includes all amino acid substitutions described as well as an additional mutation that we found to be generated at very high frequency in vitro upon PCR amplification (F33V). The nucleotide sequence of the synthetic gene was:
atgaacagcaaagacctgacgccgttcttgacgaccttgtg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com