Stabilizing compositions and methods for extraction of ribonucleic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Protocol for Obtaining Saliva Samples from Human Subjects
[0108]The subject is instructed to wait for a period of 30-60 minutes before last eating. If possible, the subject will brush his teeth (without using toothpaste). If possible, the subject will rinse his / her mouth with 50 ml of water. The subject will be requested to wait for 5-10 minutes to allow the mouth to clear of water. For subjects able to spit, they will be instructed to spit saliva into the special collection tube until the level of saliva reaches the 1 or 2 ml mark. Waiting after last eating and rinsing the mouth is desirable but not essential. Collection of saliva may take several minutes. If the subject finds that he / she is unable to deliver sufficient saliva, he / she will be given a few grains of table sugar to chew or place on the top of their tongue, and told not to be concerned if some of the sugar is spit into the tube. For subjects unable to spit (e.g., infants, young children, individuals with limitations / dis...
example 2
Comparison of the Present Composition to Oragene™
[0111]In this example, a single subject provided two sputum / saliva samples (2 ml each) within a short period of time. One sample was collected into a vial containing 2 ml of a composition of the present invention comprising: 4% SDS, 50 mM CDTA, pH 6.6. Shortly afterwards, the same subject provided a second sample into a vial containing 2 ml of Oragene™ solution. Samples were shaken and left at room temperature (RT) for 3 days before purification of nucleic acids.
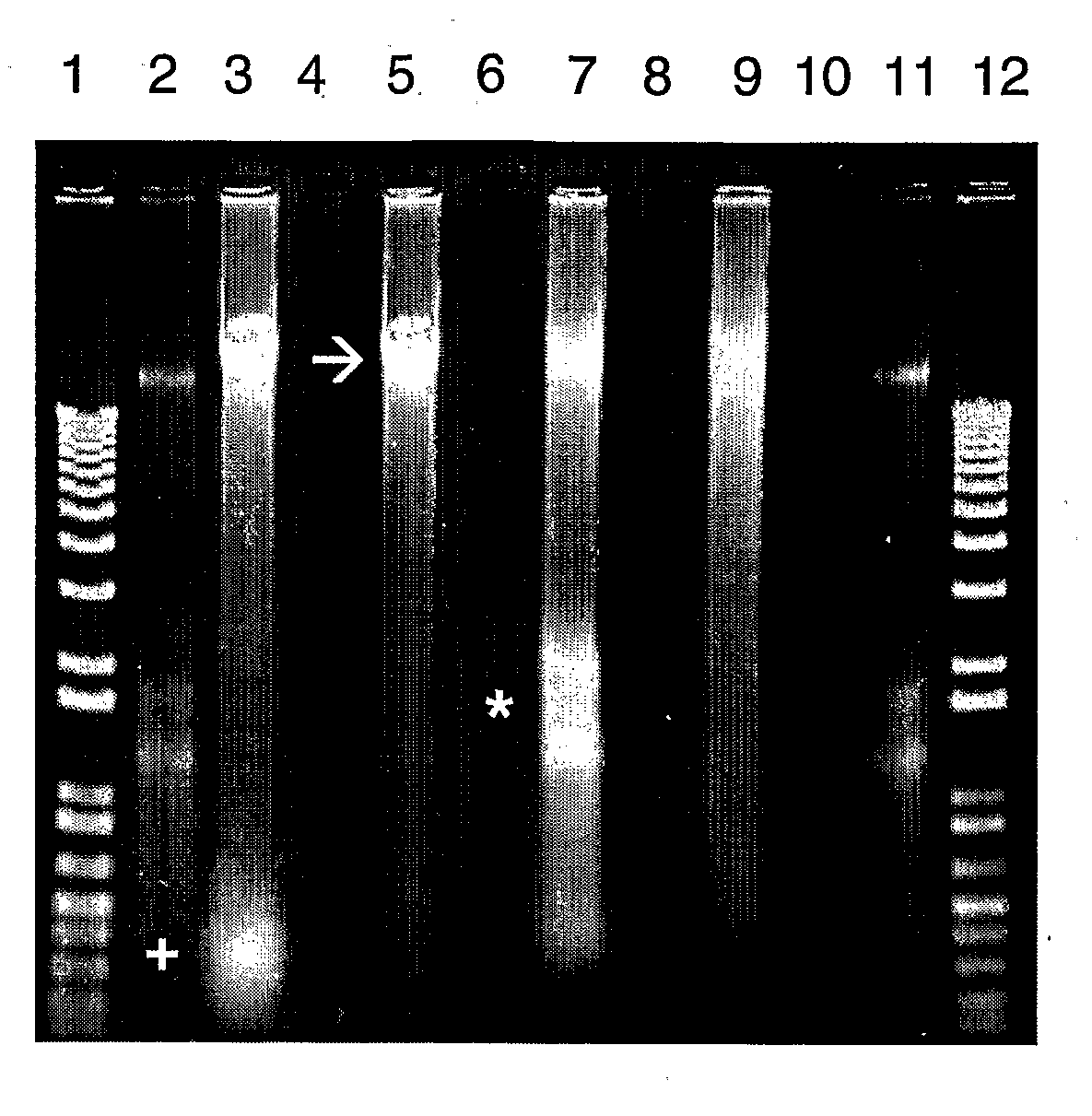
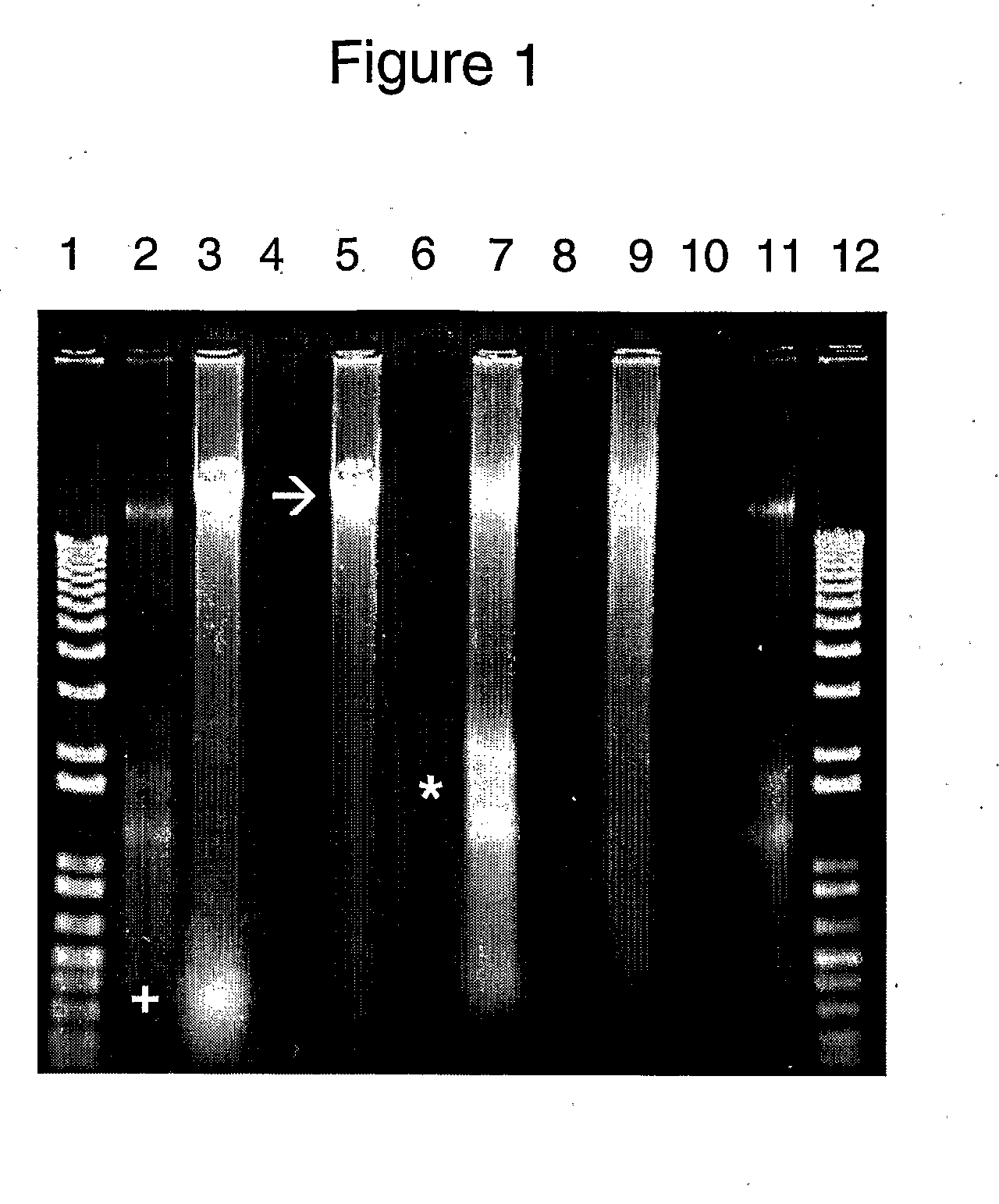
Methods
[0112]Nucleic acids were purified from this subject's saliva in Oragene™ or in the composition of the present invention. A portion of the subject's sample in Oragene™ was mixed with proteinase K and heated at 50° C. for 2 hours, without (FIGS. 1 and 2) and with (FIG. 2) an additional, subsequent heating at 90° C. for 15 minutes. A portion of the subject's sample in the composition was mixed with proteinase K, heated at 50° C. for 2 hours and then at 90°...
example 3
Optimizing pH Range for Maintaining the Stability of Pure RNA Derived from Sputum / Saliva
Methods
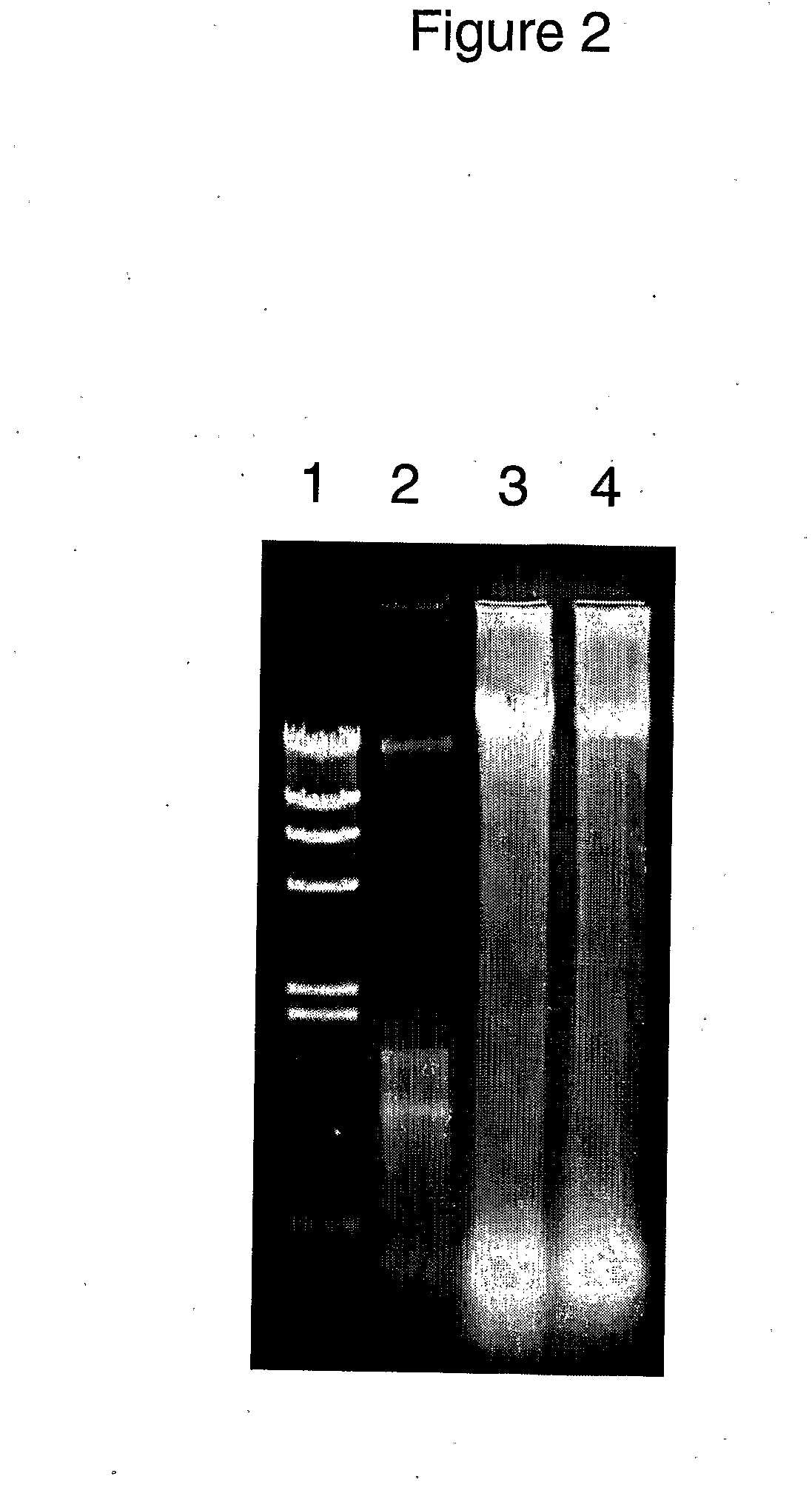
[0118]Pure RNA was diluted 6-fold into solutions buffered over a wide range of pH values (pH 3.0, 5.0, 6.0, 7.0, 8.2 and 10.0): Following a 16 hour period of incubation at 50° C., to allow partial hydrolysis of the phosphodiester backbone of the RNA to occur, an aliquot of the RNA buffered to each pH value (noted below) was analyzed by electrophoresis on a 0.9% agarose gel and stained with ethidium bromide (FIG. 3).
[0119]The sample order of FIG. 3 is as follows:
LaneSample1Lambda DNAHindIII digest2RNA marker3RNA, pH 3.04RNA, pH 5.05RNA, pH 6.06RNA, pH 7.07RNA, pH 8.28RNA, pH 10.0
Conclusions
[0120]RNA is stable chemically at neutral to slightly acid pH, as indicated by the preservation of the stained double bands characteristic of ribosomal RNA seen in treatments at pH 5.0-8.2. Note that a decrease in intensity of the upper band is expected before a decrease in the lower band. While not wishi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com