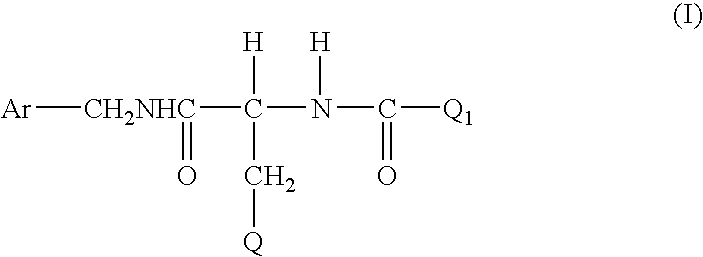
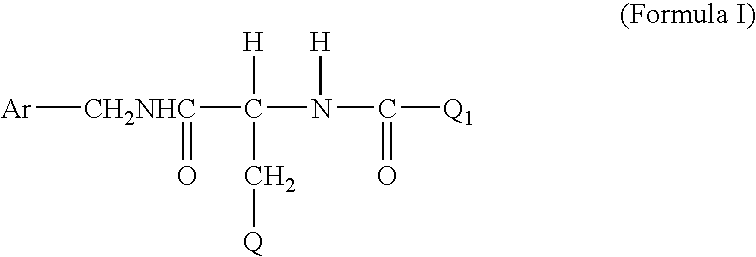
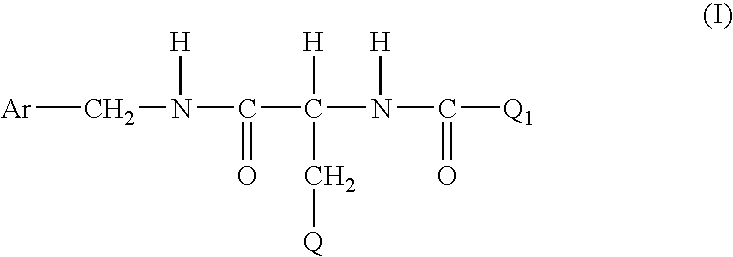
Method for treating diabetic peripheral neuropathic pain
a peripheral neuropathic pain and diabetic technology, applied in the direction of biocide, amide active ingredients, drug compositions, etc., can solve the problems of no survival advantage, no effective treatment available, increase of firing rate and starting or increasing spontaneous activity,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Chronic Constriction Injury
CCI, Bennett-Model
[0047]The effectiveness of harkoseride in reducing spontaneous chronic pain, mechanical allodynia, and thermal hyperalgesia was tested using the chronic constriction injury (CCI) model of peripheral neuropathy, one of the best characterised in vivo animal models used to study chronic pain due to peripheral nerve injury. In this model, loose ligatures are placed around the sciatic nerve, which produces axonal swelling and a partial deafferentation manifested as a significant but incomplete loss of axons in the distal portion of the peripheral nerve. One of the prominent behaviours seen following sciatic nerve ligation is the appearance of hind paw guarding, thought to be an indication of an ongoing spontaneous chronic pain. Support for this idea is derived from reports of increased spinal cord neural activity, and increased spontaneous neuronal discharge in spinothalamic tract neurons and in the ventrobasal thalamus in the absence of overt...
example 3
Tail Flick Test, Rat
[0057]Harkoseride was additionally tested for potential activity in acute spinal thermal nociception using the tail flick test. In this model of acute thermal spinal / reflex hyperalgesia radiant heat is applied to the animal's tail approximately 2 cm from the tip and time latency for withdrawal reaction is automatically assessed by an algometer, a defined maximal stimulus time prevents tissue damage. This test is widely used as an assay for the anti-nociceptive efficacy of pharmacological agents and is highly predictive of acute analgesic efficacy in humans. Usually pure analgesics of the opioid type are most active; neither adjuvants like amitryptiline nor anti-epileptics nor NSAIDs (non-steroidal anti-inflammatory drugs) are active.
[0058]Results for 20 and 40 mg / kg harkoseride i.p are shown in table 5 [percent anti-nociception, calculated as [{(post-drug latency)−(pre-drug-latency)} / {(max. latency)−(pre-drug latency)}×100]±SEM, n=12 / group]. A baseline or pre-dru...
example 4
The Anti-Nociceptive Activity of Harkoseride in Comparison with Gabapentin
[0060]In the following explained study the used harkoseride is hereinafter abbreviated as SPM 927 and gabapentin is hereinafter abbreviated as GBP.
Objective
[0061]The major aim of this study was to assess the anti-nociceptive activity of SPM 927 and gabapentin (GBP) in rodent models for inflammatory pain and to compare the in vivo effects of each drug with each other.
Methods:
[0062]Carrageenan-induced mechanical hyperalgesia in rats was induced by subplantar injection of a 0.1 ml of a 2% carrageenan suspension and measured 3 h afterwards by the paw pressure (Randall-Sellito) test.
[0063]Subchronic inflammatory nocicpetion in mice was induced by the subplantar injection of formalin (0.02 ml of a 5% solution). Nociceptive behaviour (paw licking) was measured and quantified between 0 and 5 min (acute pain) and between 20 and 30 min (subchronic inflammatory pain) after formalin.
[0064]Drugs and experimental design: SP...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| chemical name | aaaaa | aaaaa |
| enantiomers | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com