Plexin d1 as a target for tumor diagnosis and therapy
a tumor and plexin technology, applied in the direction of dna/rna fragmentation, peptide/protein ingredients, depsipeptides, etc., can solve the problems of secondary tumor cell death, inhibit the growth of infiltrative cells, and the translation to clinical level has so far been less successful, so as to inhibit the migration of tumor cells and inhibit the migration of macrophages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Specific Expression of Plexin D1 on Tumor-Associated Blood Vessels
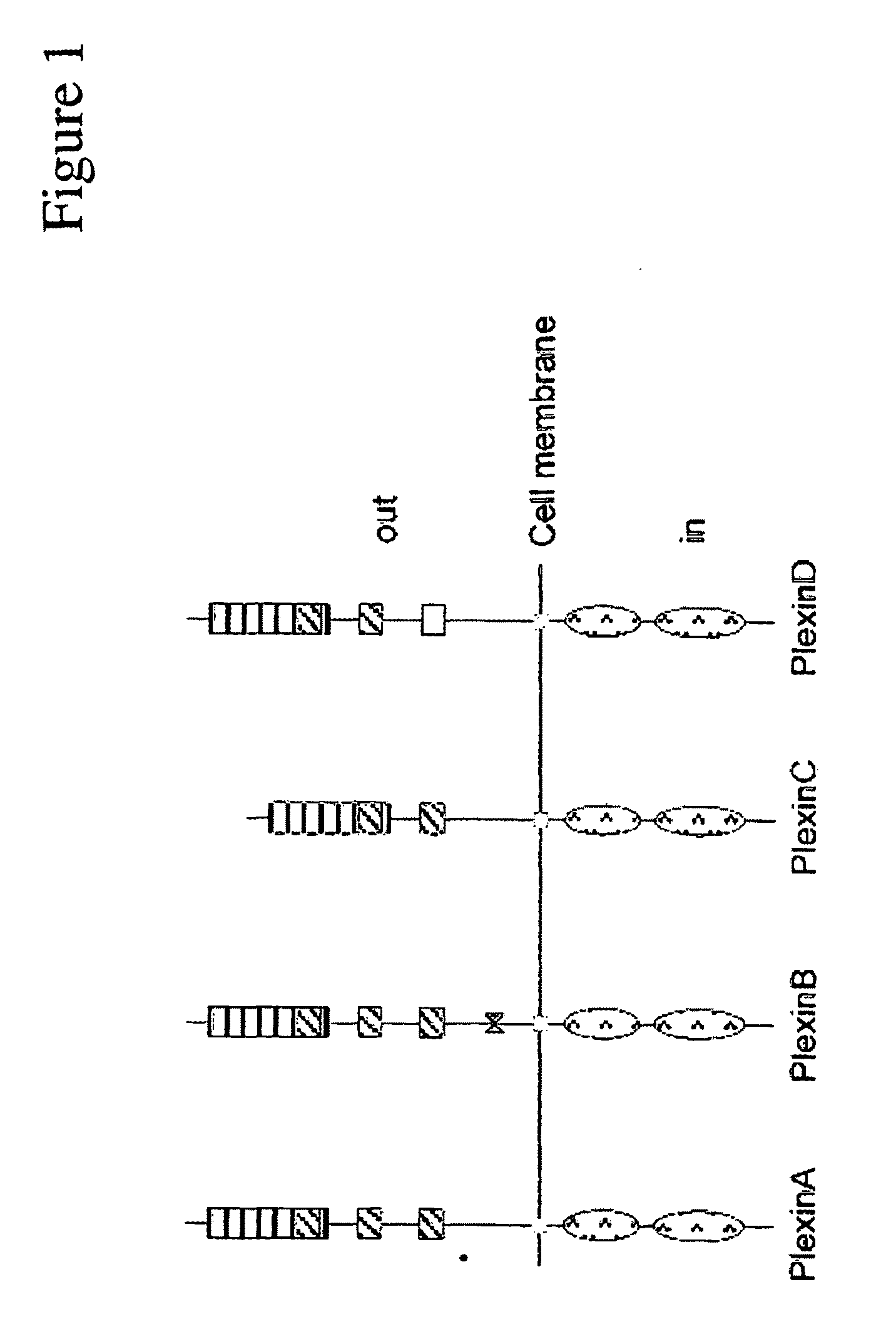
[0110]Plexin D1 is expressed on neurons but also endothelial cells in angiogenic vessels during embryogenesis. The present invention demonstrates that plexin D1 is expressed on tumor-associated blood vessels but not on normal blood vessels. This has been shown by in situ hybridization of mouse brains, containing angiogenic human melanoma lesions (FIG. 2). The animal tumor model is described in (Kusters, B et al., Cancer Res 63:5408-5413 (2003)). In short, tumor cells are injected via a microsurgical procedure in the right carotid artery, resulting in tumor growth in the parenchyma of the right brain hemisphere. After three weeks, at the onset of neurological symptoms, mice are sacrificed and brains removed and fixed in formalin.
[0111]Sections of 4μm were subjected to in situ hybridization with digoxigenin-labeled sense and antisense RNA fragments. RNA probes were generated by transcription using T3 and T7 RNA polymera...
example 2
Expression of Plexin D1 in Tumors
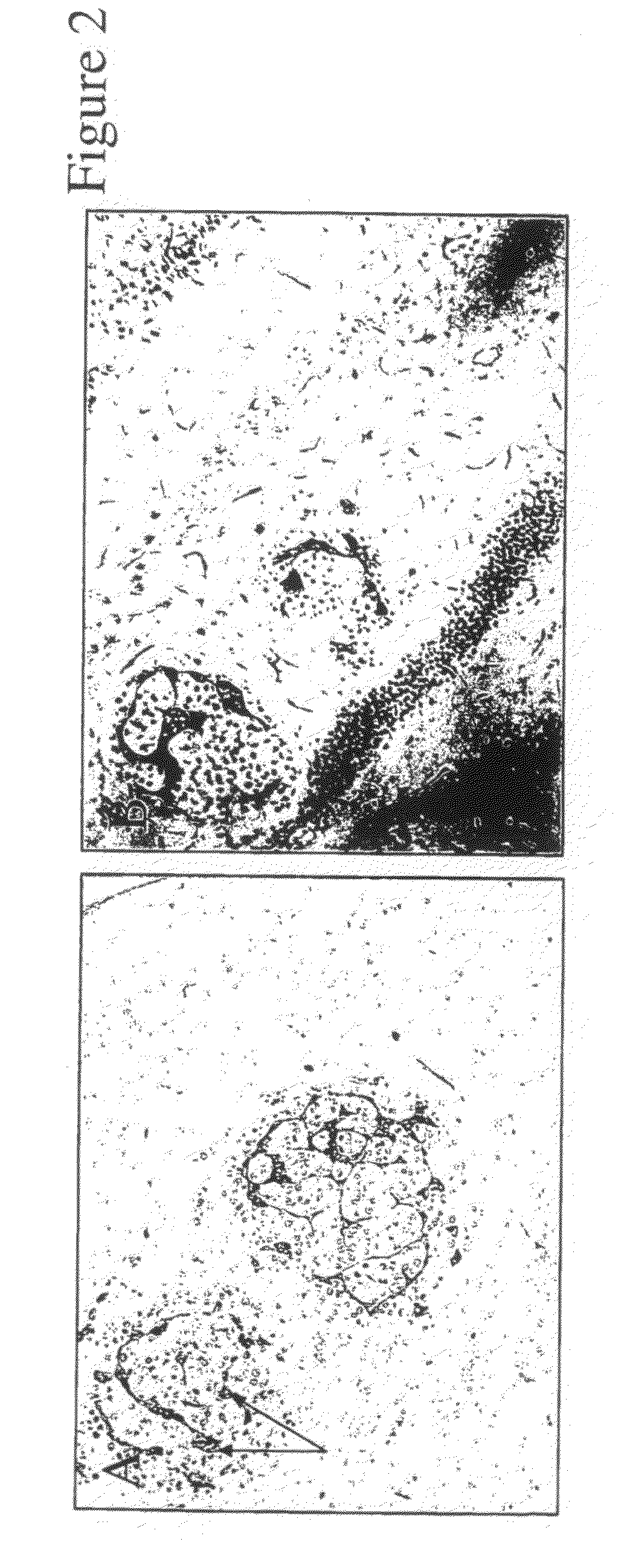
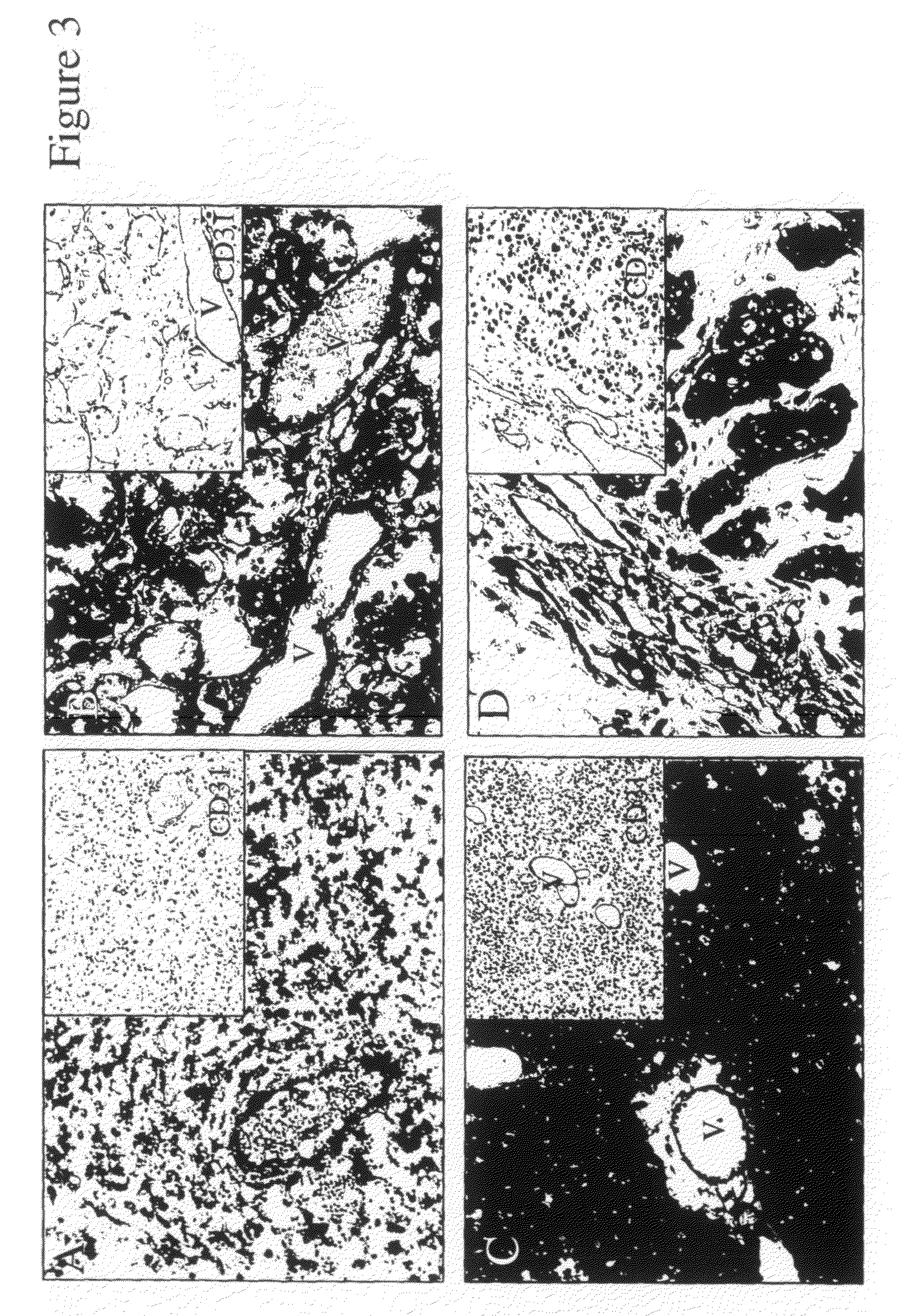
[0114]To investigate plexin D1 RNA expression in human tumor samples, we performed in situ hybridizations with a human-specific plexin D1 RNA probe. High plexin D1 RNA expression levels were found in a number of human tumors, of which (glioblastoma multiforme, brain metastases of sarcoma, renal cell carcinoma, adenocarcinoma of the colon and of the breast), both in tumor vasculature and tumor cells. A summary of plexin D1-expressing tumor types is given in Table 1. FIG. 3 shows some examples of in situ hybridizations, e.g. a glioblastoma, a brain metastasis of melanoma and a brain metastasis of colon carcinoma. Plexin D1 RNA was found not only on the tumor vasculature, but also excessively on the tumor cells themselves. Importantly as in FIG. 4A, no plexin D1 RNA expression is observed in normal brain vasculature. In FIG. 4B a CD31 staining is shown, demonstrating that abundant vessels are present in these sections.
example 3
Preparation of Antibodies Against Plexin D1
[0115]To detect plexin D1 protein, antibodies were selected with affinity towards plexin D1. To this end, a M13 pHENIX phage library was constructed expressing Llama single domain V-H antibodies, constructed by RT-PCR from Llama B-lymphocytes as described (van Koningsbruggen, S et al., J Immunol Methods 279:149-161 (2003)). The population of resulting cDNAs encoding V-H-single domain antibody (sdab) fragments was ligated into phagemid vector pHENIXHis8VSV (results not shown), resulting in a fusion product with a 8*His-tag and a VSV-G-tag at the C-terminus. After electroporation in E. coli TG1 cells, ampicillin-resistant colonies were collected and pooled.
[0116]The resulting library had a complexity of 8×108 clones. Eighty percent of plasmids contained full-length sdab insert as determined by PCR analysis and immunological dot-blot-detection of the VSV-G-tag in sdabs (see below). The phage library was propagated as phagemids in E. coli TG1 b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
| Fluorescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com