Ngna compositions and methods of use
a composition and ngna technology, applied in the field of ngna compositions and methods of use, can solve the problems of increasing the chance of viruses spreading from person to person, difficult to assess the importance of coronaviruses as causative agents, and people spending more time indoors, so as to prevent the onset of colds, improve energy, and reduce the symptoms of colds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0102]A sports drink containing NGNA was formulated for use by humans. The NGNA was made from glucosamine residues derived from crab chitin. The finished drink (per 500 ml bottle) contained: pure water; 1% Glycerol / (0, 1-10%); lemon flavor; potassium sorbate / sodium benzoate as stabilizers; and 2 mg of 99% pure NGNA.
example 2
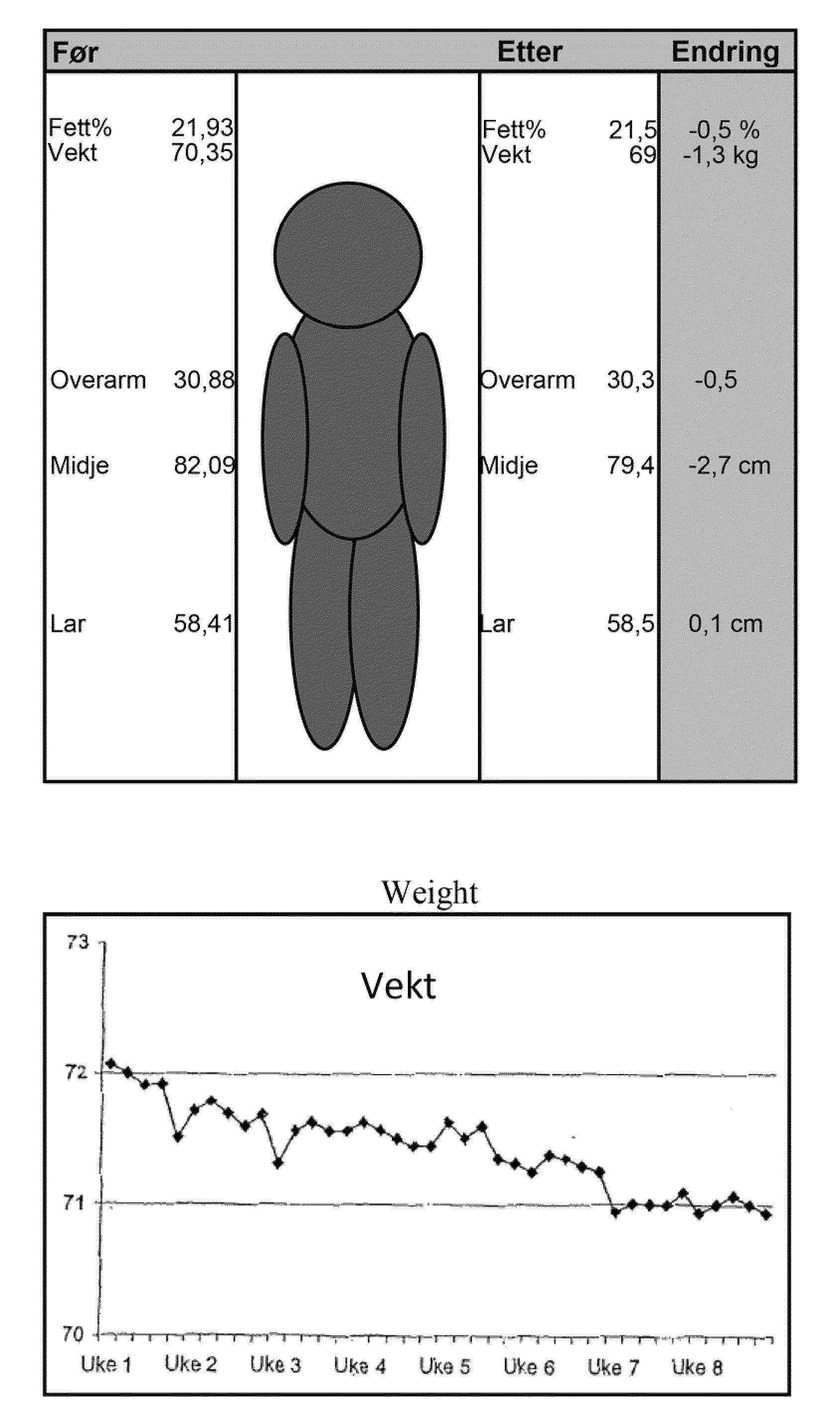
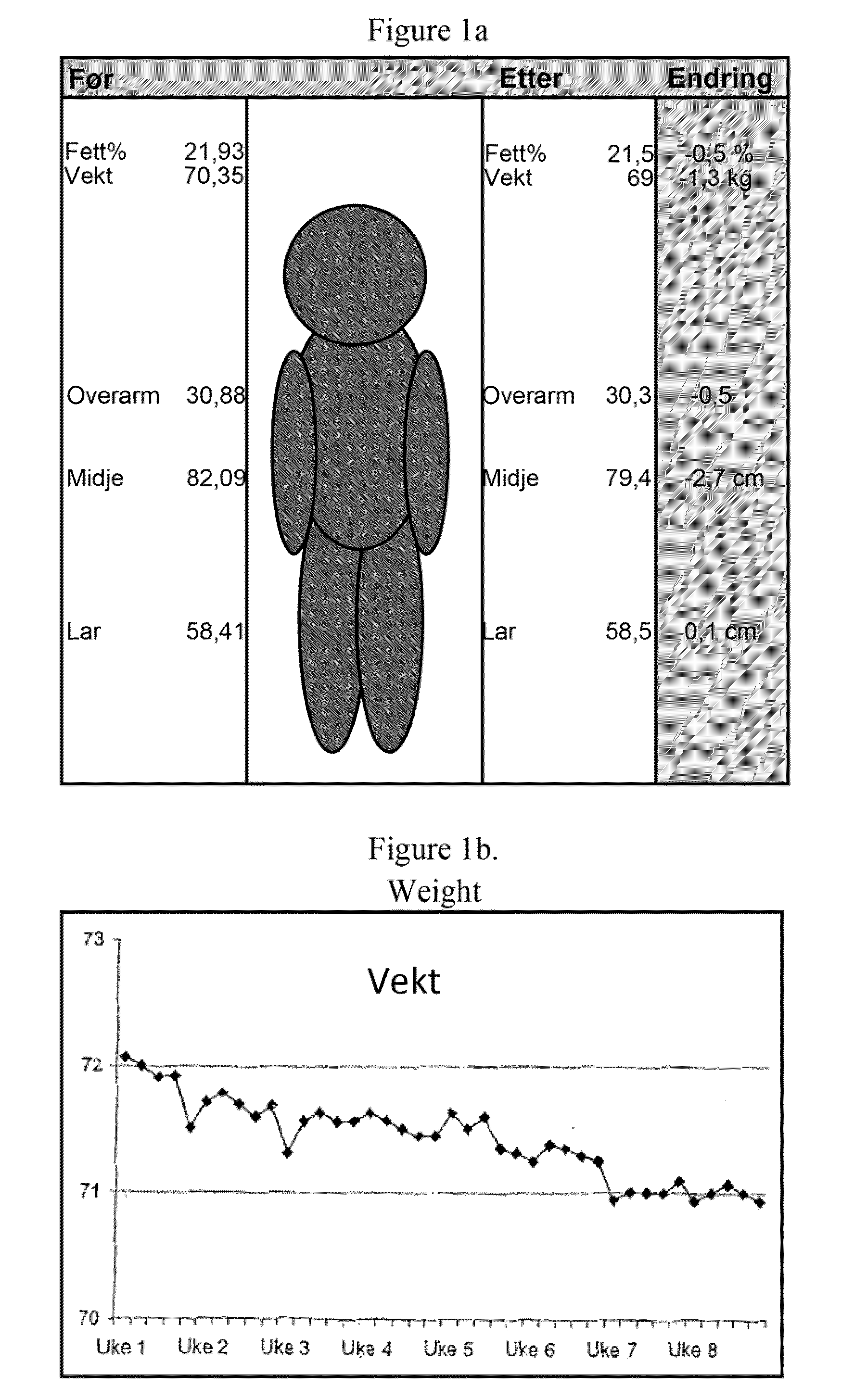
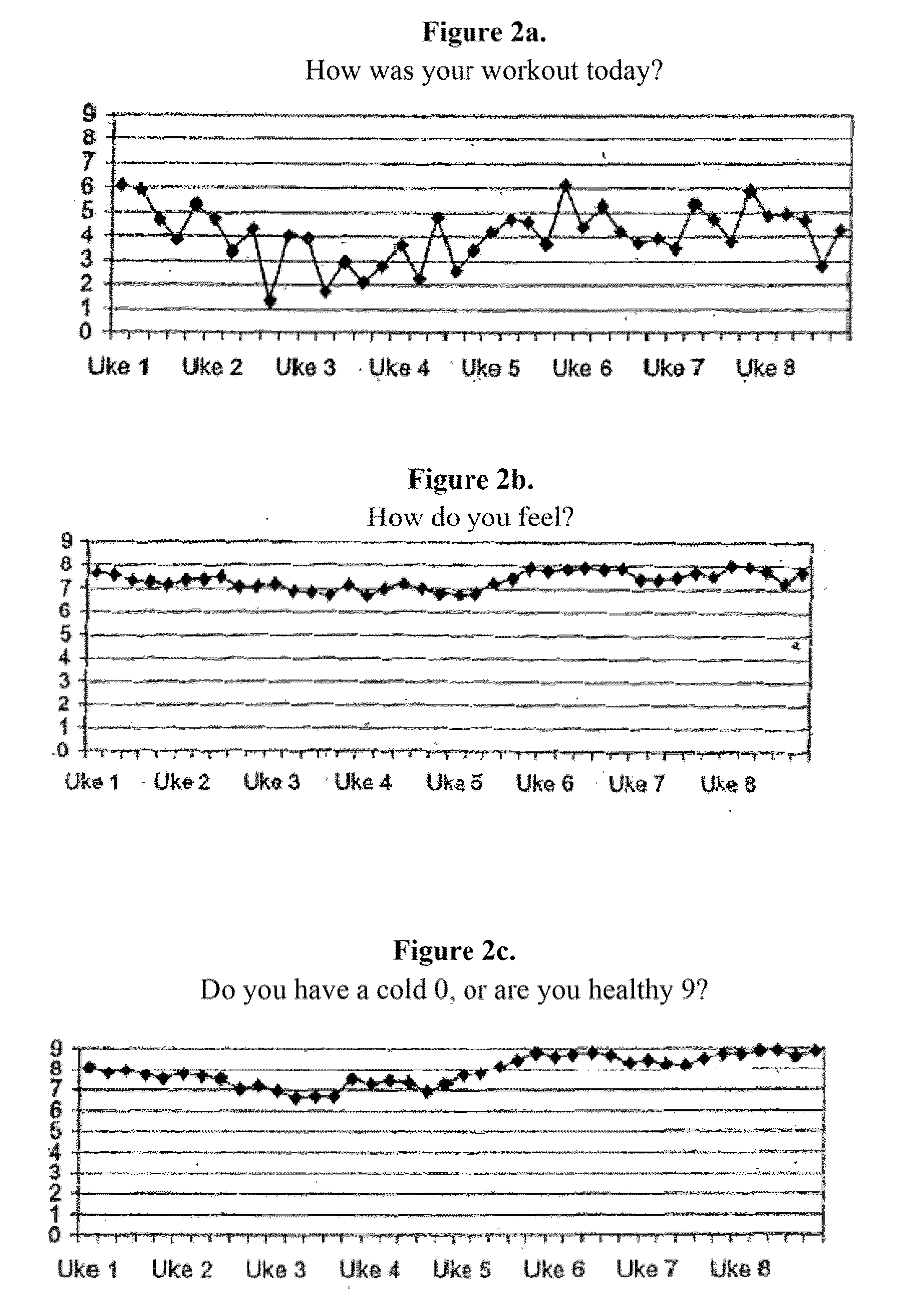
[0103]The sports drink described in Example 1 was tested in a group of 18 subjects. The subjects were supplied one 500 ml bottle of the sport drink containing 2 mg NGNA. The drink was administered during exercise or during the day when not exercising over an eight week period. The subjects worked out an average of about four times per week. The following measurements were taken during the study: fett / fat %, vekt / weight, overarm / upper arm in cm, midje / waist in cm, l∪r / thighs in cm. These results are summarized in FIG. 1A. As can be seen, there was a steady decrease in weight during the study (FIG. 1B). The subjects were also asked to evaluate other physiological effects of the drink. The subjects were asked to rank the impact of the drink on a scale of 0 to 9 (with 9 being a positive impact) with respect to the following questions:
[0104]a. How was your workout today
[0105]b. How do you feel?
[0106]c. Do you have a cold 0, or are you healthy 9?
[0107]d. Do you feel the drink helps you?
[0...
example 3
[0110]This example describes the in vitro inhibition of various viruses by compositions comprising NGNA.
Influenza
[0111]The Influenza B virus that will be used for this study will be B / Malaysia.[0112]Source: Retroscreen Virology Ltd. Virus B / Malaysia / 2506 / 2004[0113]Physical form: Virus in liquid medium[0114]Expiry date: 4 hours after dilution[0115]Storage conditions: On ice[0116]Safety precautions: Biosafety Level 2 containment conditions
[0117]The cell line to be used in Phase A is MDCK cells. The Growth Media for the MDCK cell line is DMEM, 2 mM L-Glutamine, 10% FBS (foetal bovine serum) and 2 mM Hepes buffer. The Infection Media for the MDCK cell line is DMEM, 2 mM L-Glutamine, 2 mM Hepes buffer and 1.5 mM Trypsin (TPCK treated).
[0118]In the virucidal assay the Influenza B virus titrations will be incubated for 2 to 3 days before the efficacy of the test article on the virus will be determined by HA (Haemagglutination Assay). This assay utilizes the ability of influenza virus to bi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com