Microfluidic Devices and Methods of Use in The Formation and Control of Nanoreactors
a microfluidic device and nanoreactor technology, applied in the field of fluidic species system and method control, can solve the problems of limiting the hts capacity, limiting the smallest volume of reagent that can effectively be used, and surface adsorption of reactants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
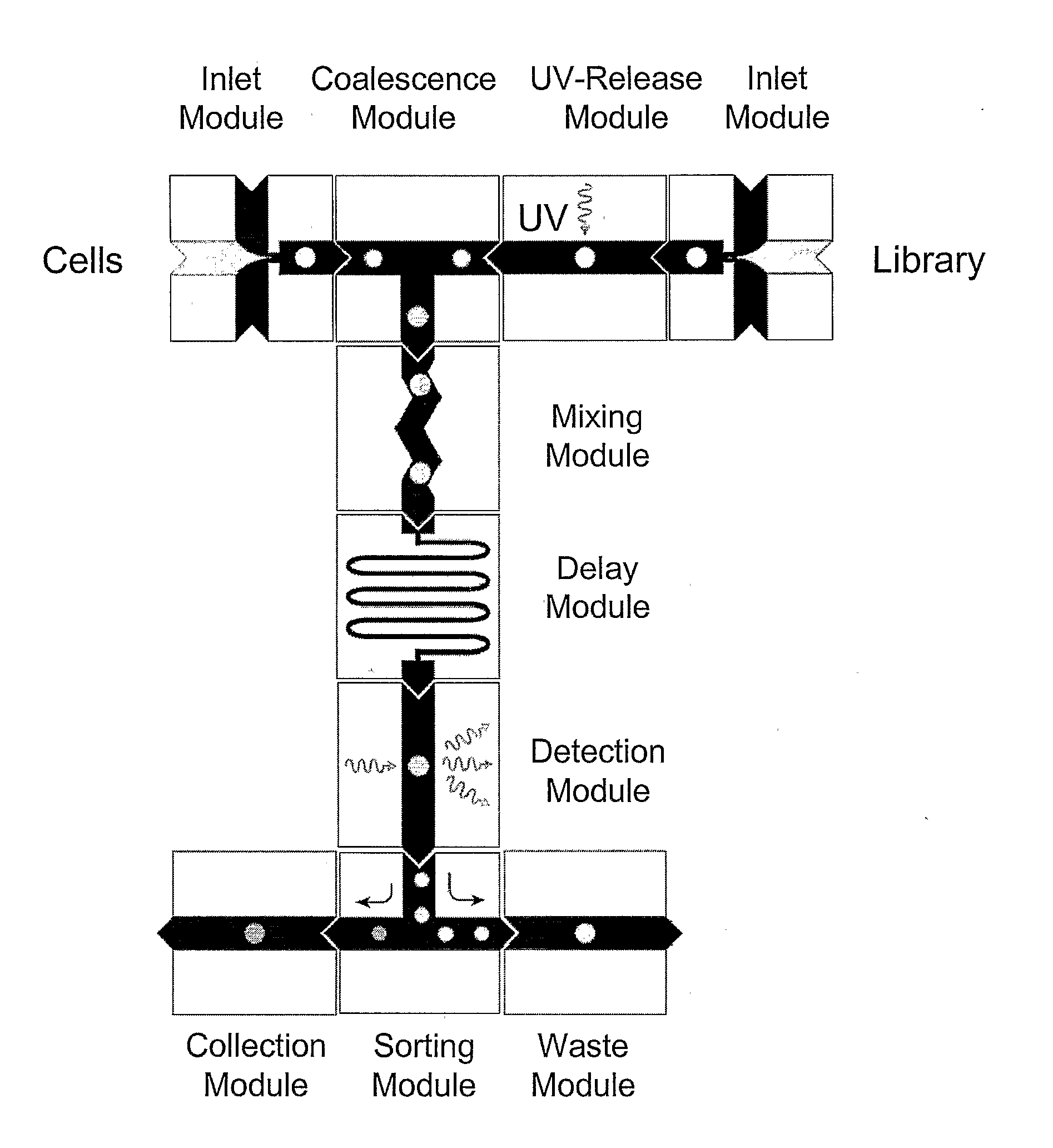
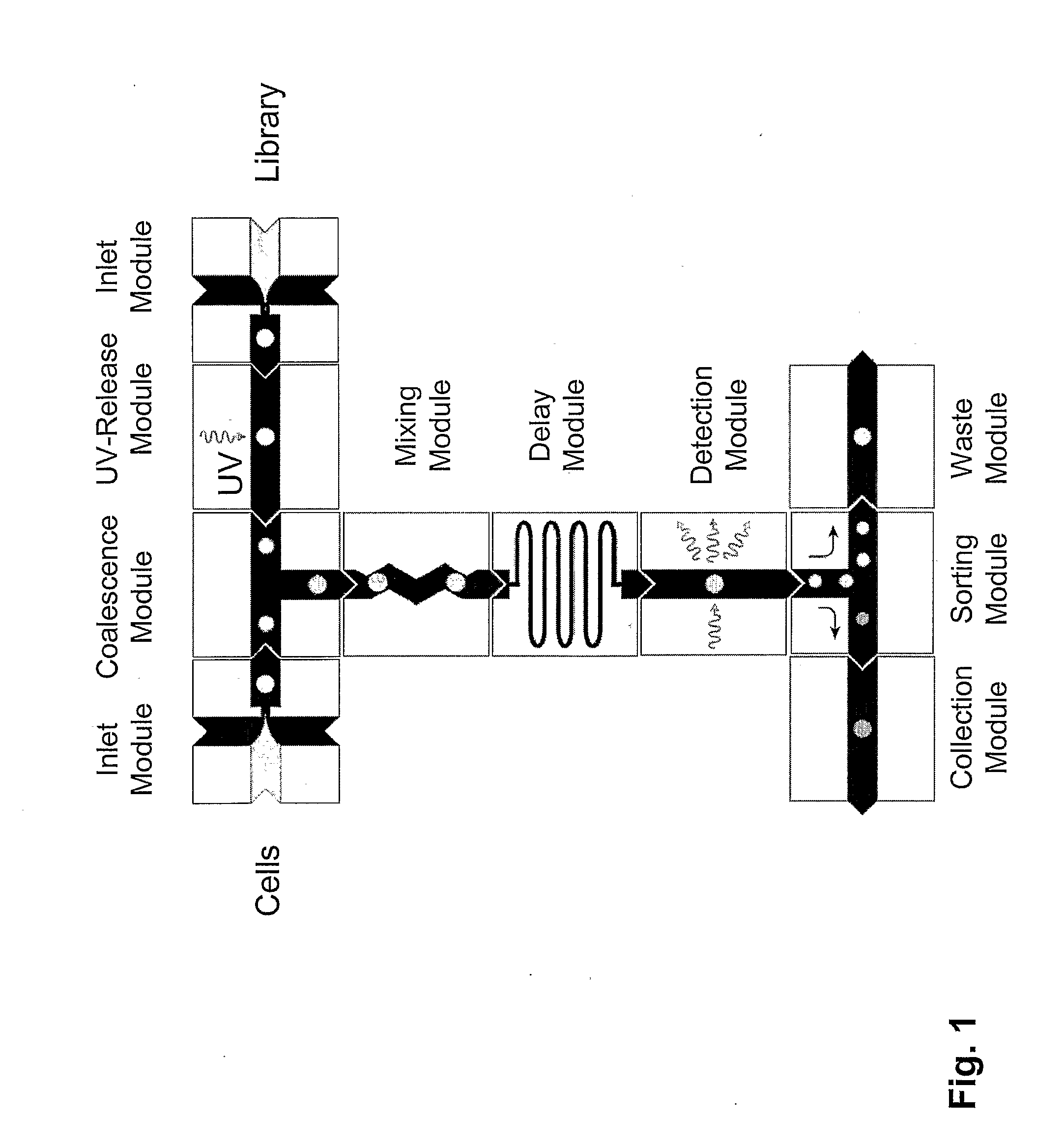
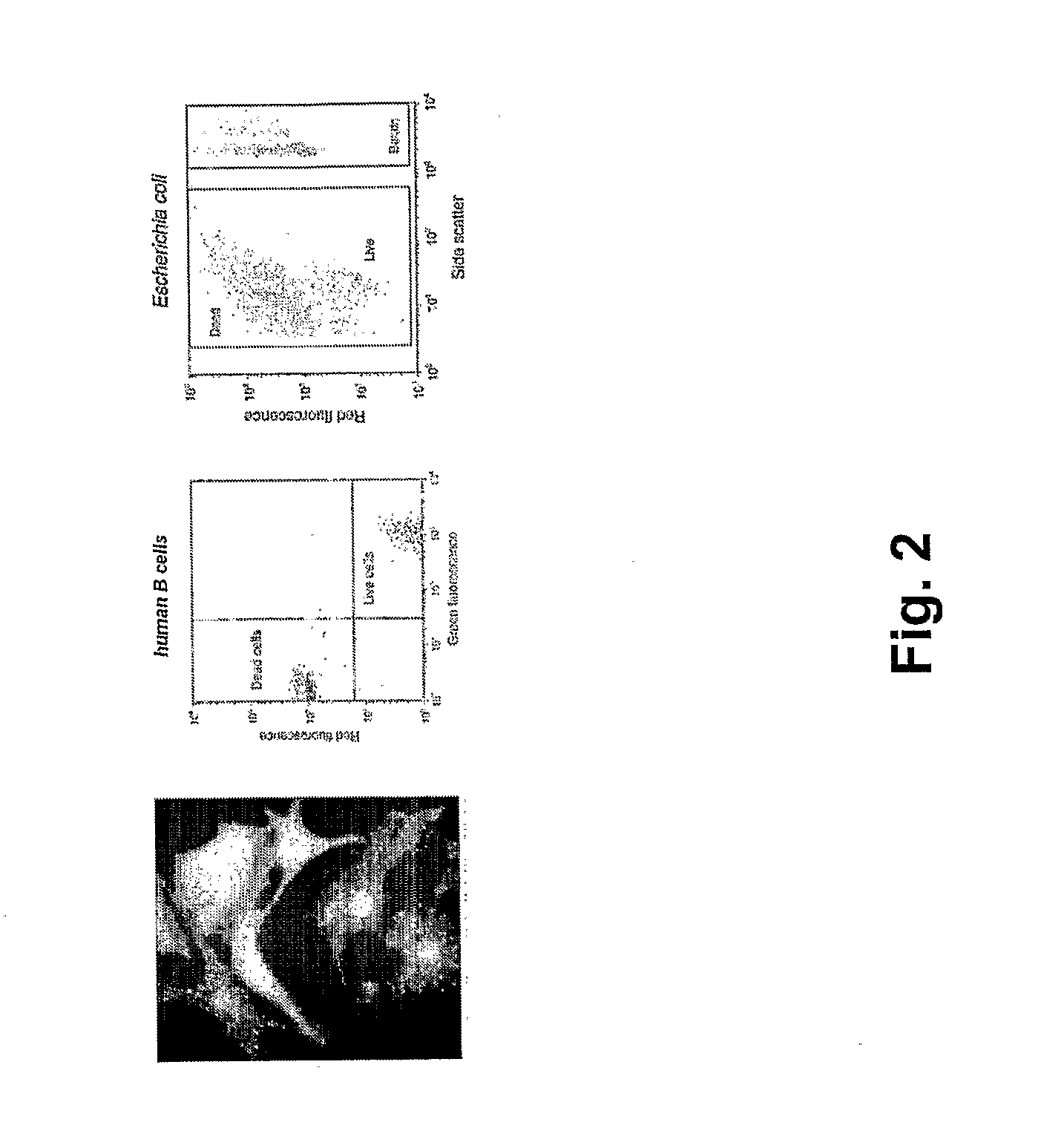
[0208]The device of the present invention can be used for Live / Dead Cell Based Assays. In one example, the assay uses two fluorophores; one is permeable across cell membranes, and a second dye binds DNA and can enter the cell only if the membrane is compromised. Similar Live / Dead assays exist for bacteria and yeast. Tagged chemical libraries and photocleavable linkers can be used in such assays. Combinatorial one-bead-one-compound libraries obtained through split-bead synthesis require a tag which describes their synthetic history in order to identify the compound reliably. Several encoding technologies for microcarriers such as beads, rods and crowns have been developed over the last decade to address this need. A simple and effective method relies on spectrometric chemical tags which are generated in parallel to the chemical entity of interested utilizing orthogonal chemistry. Alternatives include the use of nucleic acids such as DNA, followed by the use of the polymerase chain re...
example 2
[0229]The present invention provides methods for performing polymerase chain reaction in nanoreactors of the present invention as described. PCR can be performed on a drop-by-drop basis in a microfluidic device according to the present invention. A monolithic chip can be provided wherein the heating and cooling lines are built into the chip and a sorting means is provided. Advantages of performing PCR in droplets on such a chip are that the chip is disposable and the reaction can be repeated without cleaning the device between reactions. Furthermore, the chip provides a convenient way of getting all the components to perform PCR in the droplets in the right concentration. Additionally, the PCR is more efficient because the heat transfer is more efficient due to the small volume. This provides for shorter incubation / residence times. Droplets containing the nucleic acids, all PCR primers, and, if present, beads are generated one at a time at rates between 100 and 20,000 droplets per s...
example 3
[0234]The device of the present invention can be used to screen chemical libraries composed of at least 106 molecules against an established cell line. In this manner, positive and negative nanoreactors can be tracked and sorted using either a nucleic-acid based, or multi-colored bead-based encoding scheme For example, a control library with known hits can be screened against a human cancer cell line.
[0235]In one embodiment, a chemical library can be screened using a nanoreactor as described in detail herein. The power of the present invention comes from a combination of compartmentalization and electrical manipulation that enables multi-step chemical processing, including analysis and sorting, to be initiated in confinement with exquisite timing and metering precision. This multi-step processing of isolated components is essential for searching through molecular libraries for rare interactions with cells, nucleic acids, enzymes, coded microbeads, and other biomaterials. For example...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com