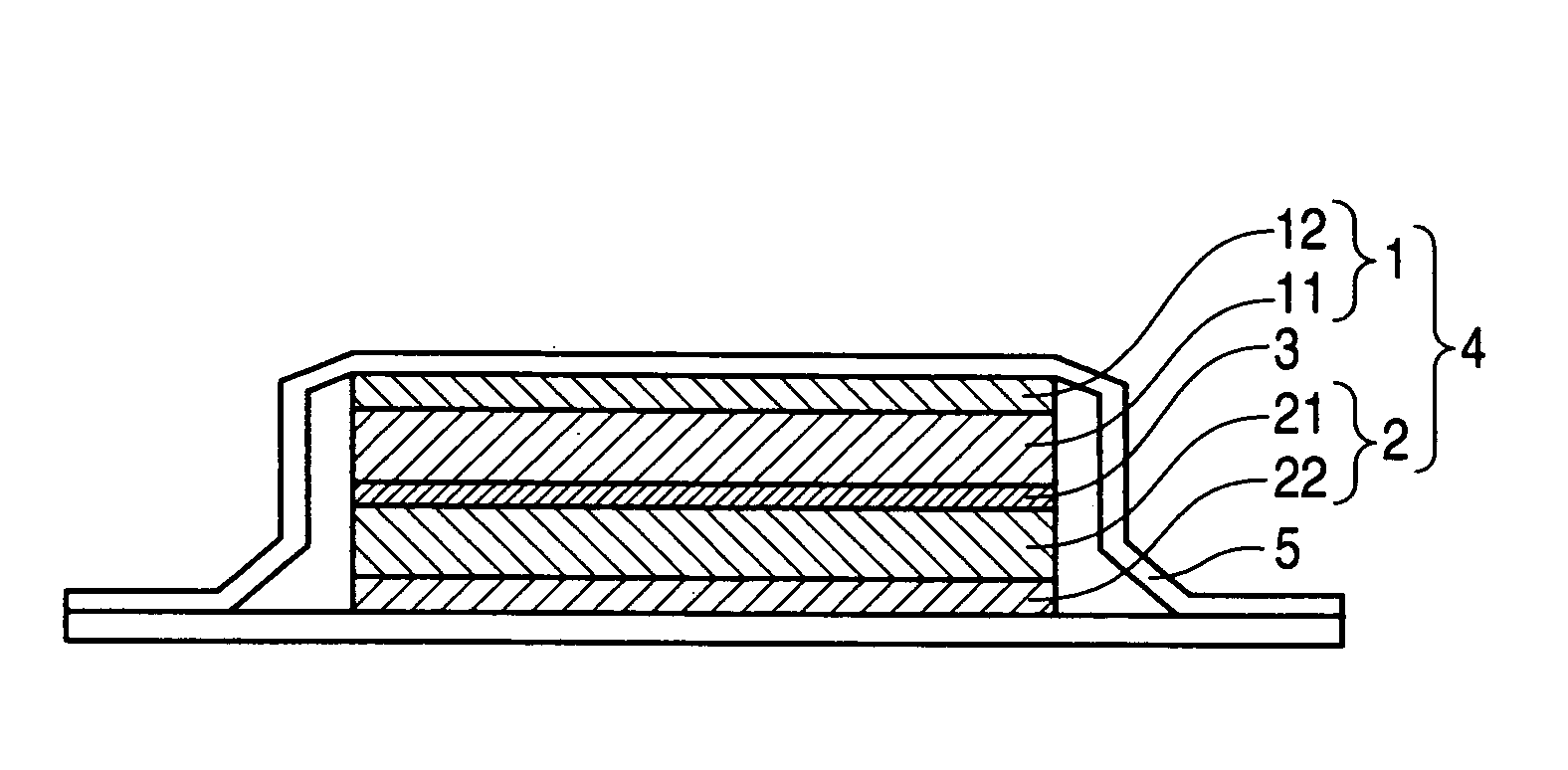
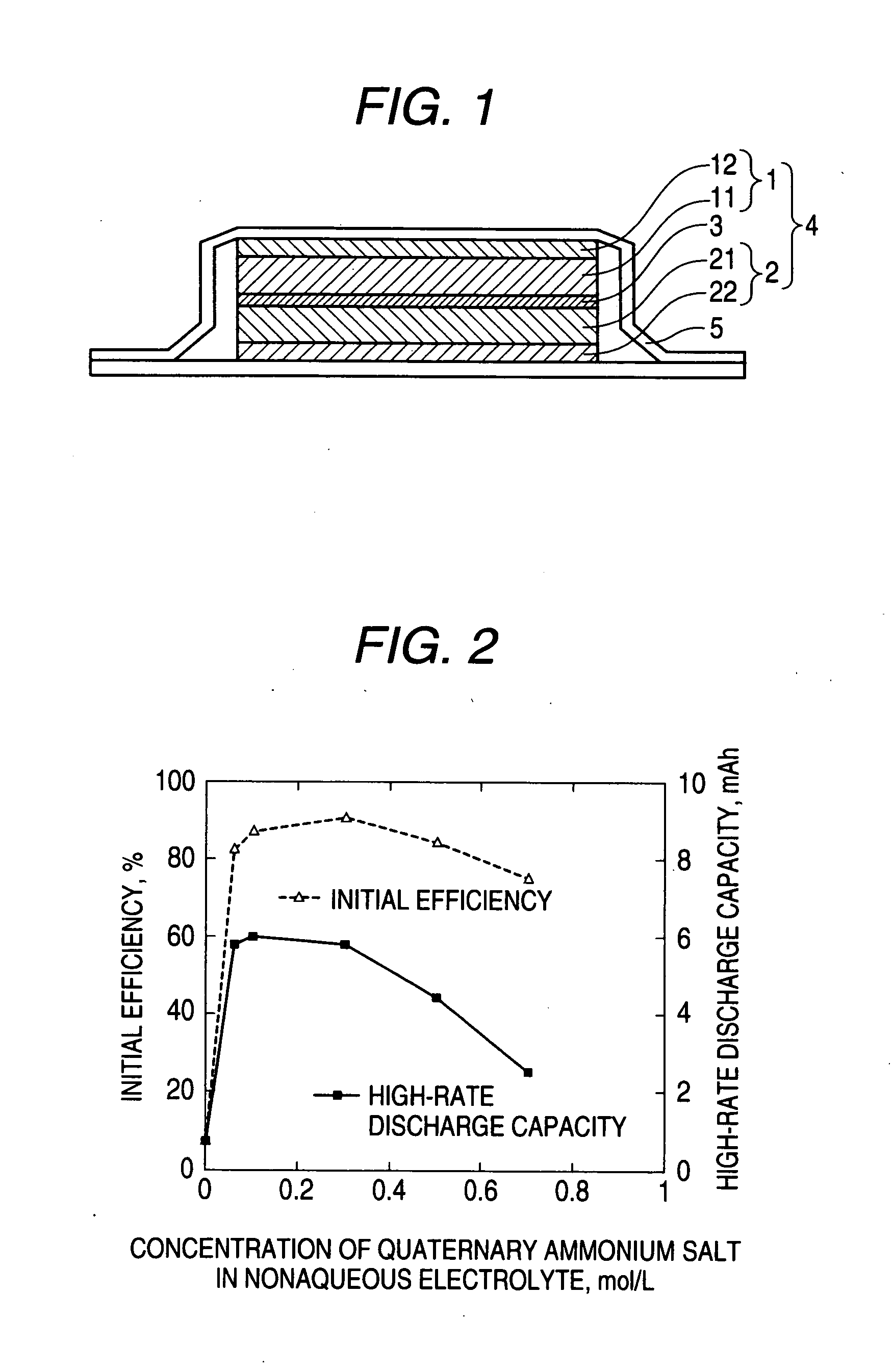
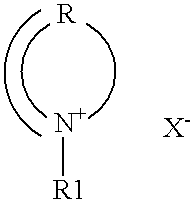Nonaqueous electrolyte and nonaqueous-electrolyte battery
a nonaqueous electrolyte and battery technology, applied in the direction of non-aqueous electrolyte cells, cell components, electrochemical generators, etc., can solve the problems of poor oxidation resistance, insufficient inhibition effect of technique, and inability to conduct charge/discharge at a high efficiency, so as to achieve effective inhibition of decomposition and improve the high-rate discharge characteristics , the effect of high energy density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0078]One mole of LiPF6 was dissolved in 1 L of a mixed solvent prepared by mixing ethylene carbonate, propylene carbonate, and diethyl carbonate in a ratio of 6:2:2 by volume. Furthermore, tetraethylammonium tetrafluoroborate ((C2H5)4NBF4) was mixed therewith in an amount of 0.06 mol / L. Thus, a nonaqueous electrolyte was obtained.
example 2
[0079]One mole of LiPF6 was dissolved in 1 L of a mixed solvent prepared by mixing ethylene carbonate, propylene carbonate, and diethyl carbonate in a ratio of 6:2:2 by volume. Furthermore, trimethyl-n-butylammonium bis(trifluoromethylsulfonyl)imide (CH3)3(n-C4H9)N(CF3SO2)2N) was mixed therewith in an amount of 0.1 mol / L. Thus, a nonaqueous electrolyte was obtained.
example 3
[0080]One mole of LiPF6 was dissolved in 1 L of a mixed solvent prepared by mixing ethylene carbonate, propylene carbonate, and diethyl carbonate in a ratio of 6:2:2 by volume. Furthermore, 1-ethyl-3-methylimidazolium bis(perfluoroethylsulfonyl)imide was mixed therewith in an amount of 0.3 mol / L. Thus, a nonaqueous electrolyte was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com