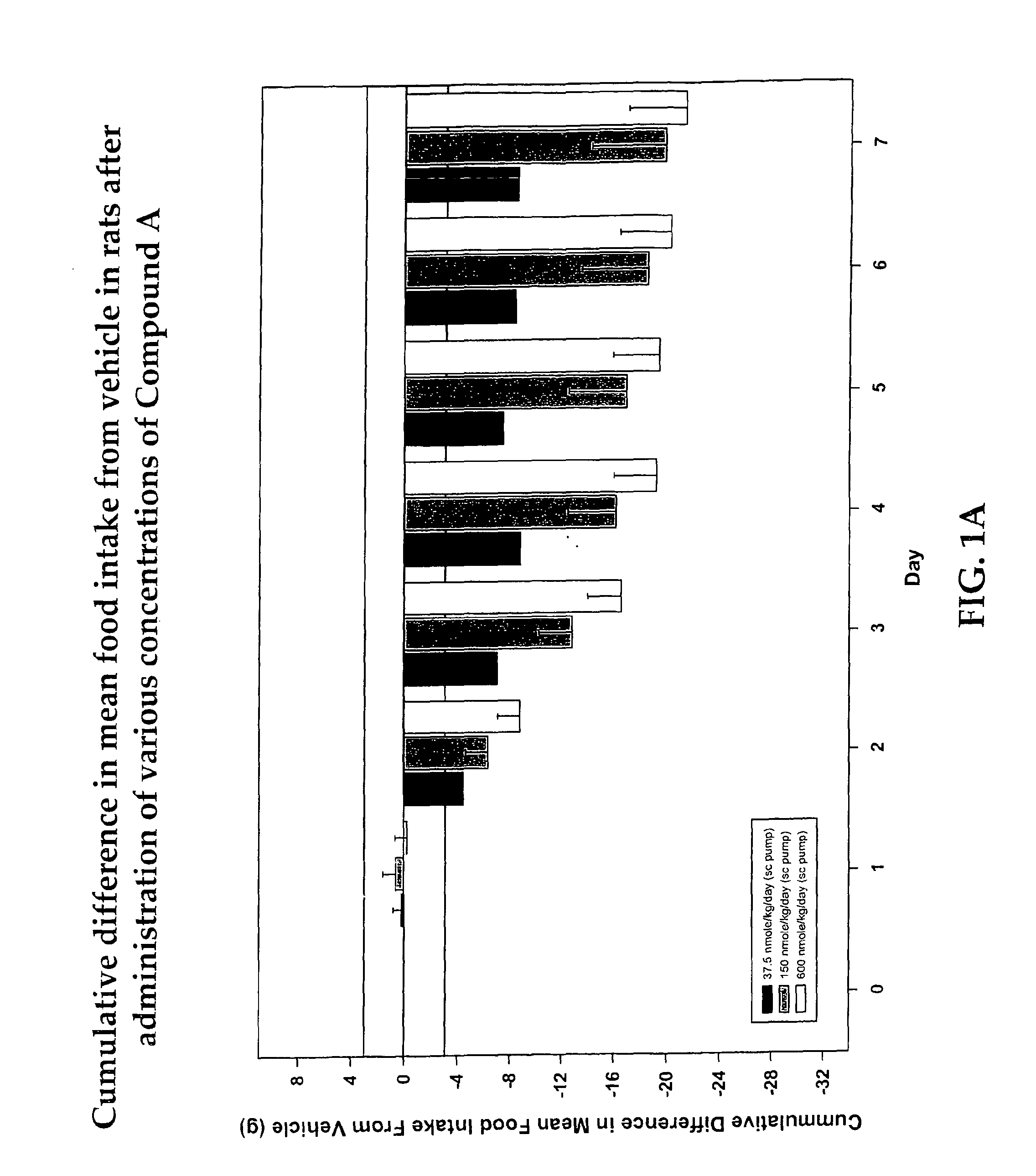
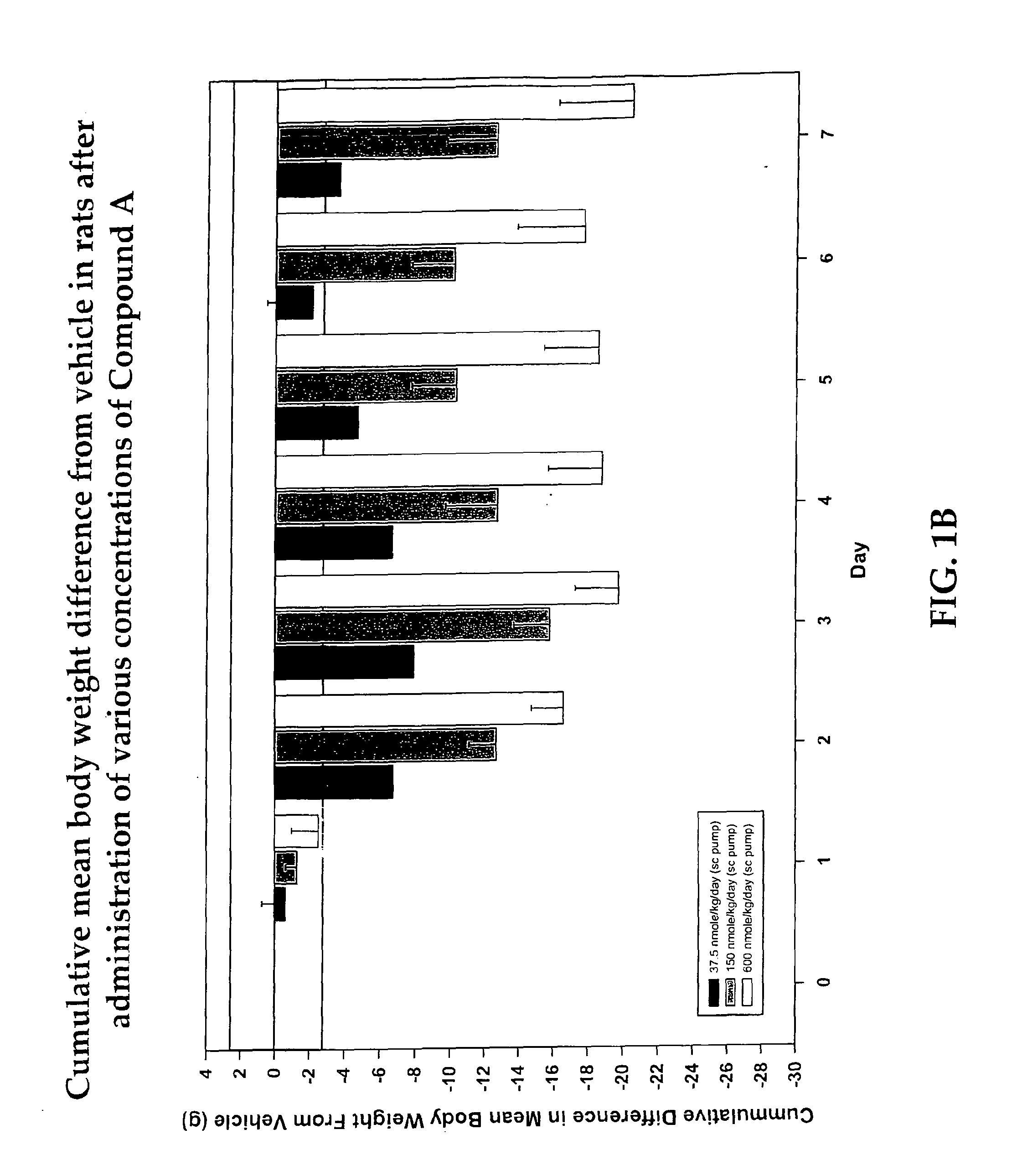
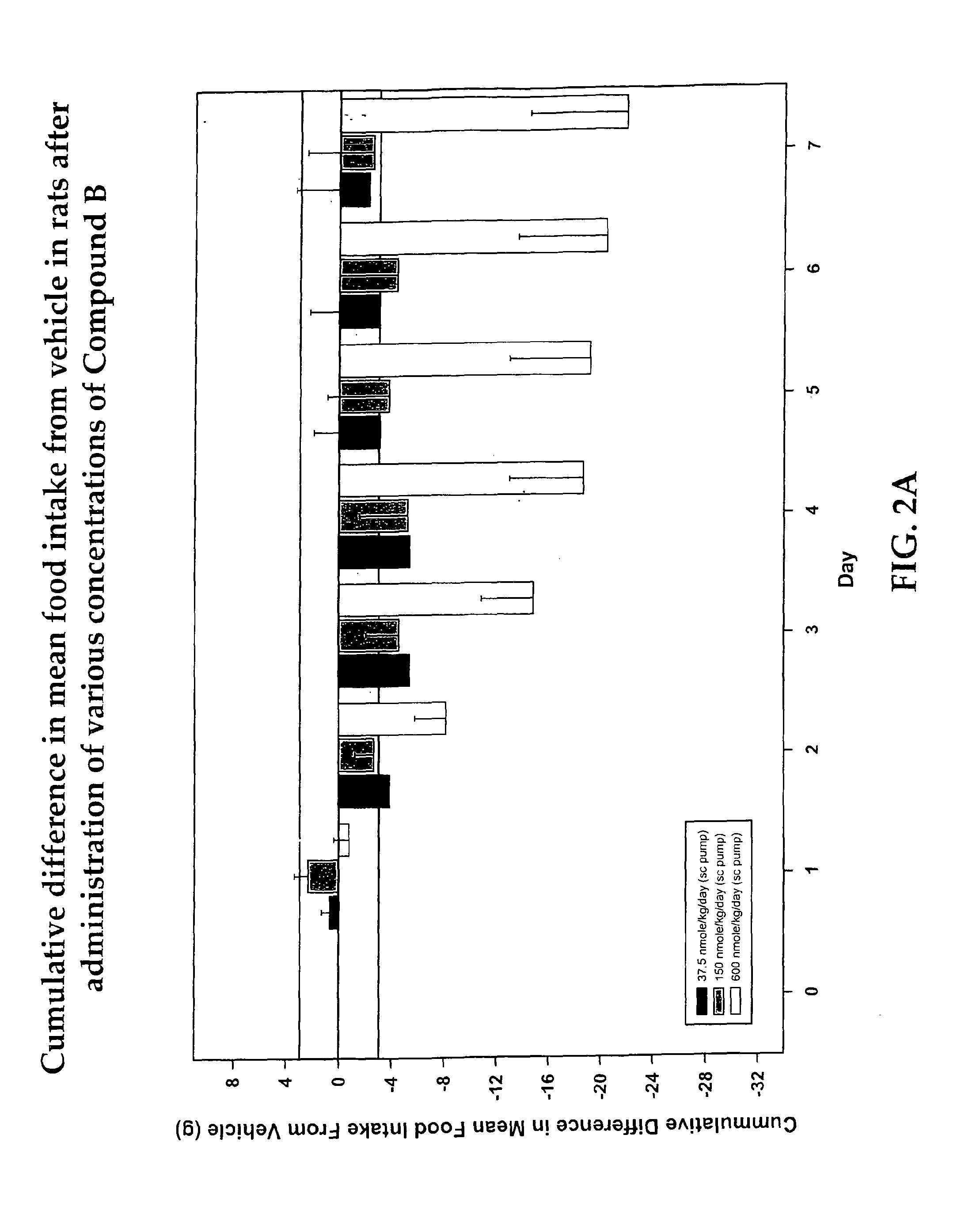
Cyclic peptide melanocortin receptor ligands
a technology of melanocortin receptor and cyclic peptide, which is applied in the field of peptides, can solve the problems of increasing the risk of skin cancer and adrenal tissue carcinoma, and achieve the effect of reducing alcohol consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
H-His-Arg-c(Cys-D-Ala-His-D-Phe-Arg-Trp-Cys)-NH2 (SEQ ID NO.:2)
[0145]The peptide was assembled using Fmoc-chemistry on an ABI 433A peptide synthesizer (Applied Biosystems, Foster City, Calif.) at the 1.0 mmole scale. The reaction vessel containing 1350 mg of 0.74 mmol / Rink Amide MBHA resin (Novabiochem, San Diego, Calif.) was placed in a reaction vessel. The resin was then treated with 10 ml of NMP for 15 min to swell the resin. The ABI FastMoc 1.0® protocol was used to generate the peptide. Each cycle comprised of deblocking the N-terminal Fmoc using 20% piperidine followed by extensive NMP washing. Pre-packaged 1.0 mmole cartridge of each amino acid was then dissolved in 0.45 M HOBT / HBTU and transferred to the activation vessel. Two more 1.0 mmole amino acid cartridges were dissolved and transferred to the activation vessel for a total of 3 equivalents of amino acid used per coupling step. A 3 ml of a 2 M DIPEA solution, was then introduced to the activation vessel for a total of ...
example 2
Ac-2-Nal-Arg-c(Cys-D-Ala-His-D-Phe-Arg-Trp-Cys)-NH2 (SEQ ID NO.:1)
[0147]The peptide was assembled using Fmoc-chemistry on an ABI 433A® peptide synthesizer (Applied Biosystems; Foster City, Calif.) at the 1.0 mmole scale. Approximately 1350 mg of 0.74 mmol / Rink Amide MBHA resin (Novabiochem®, San Diego, Calif.) was placed in a reaction vessel. To swell the resin, it was treated with 10 ml of NMP for 15 minutes. The ABI FastMoc 1.0 protocol was used to generate the peptide. A cycle comprised of deblocking the N-terminal Fmoc using 20% piperidine followed by NMP washing. A 1.0 mmole cartridge of each amino acid was dissolved in 0.45M HOBT / HBTU and transferred to the activation vessel. Two additional 1.0 mmole amino acid cartridges were then dissolved and transferred to the activation vessel for a total of 3 equivalents of amino acid per coupling step. Approximately 3 ml of a 2 M DIPEA solution was introduced to the activation vessel resulting in 6 equivalents contained therein. The res...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com