Metallocenyl Phthalocyanine Compounds and Use Thereof
a technology of phthalocyanine and phthalocyanine, which is applied in the field of metalocenyl phthalocyanine compounds and derivatives, can solve the problems of increasing writing power, limiting the maximum achievable writing speed at a given laser point, and jitter value, and achieves excellent recording sensitivity, small jitter, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
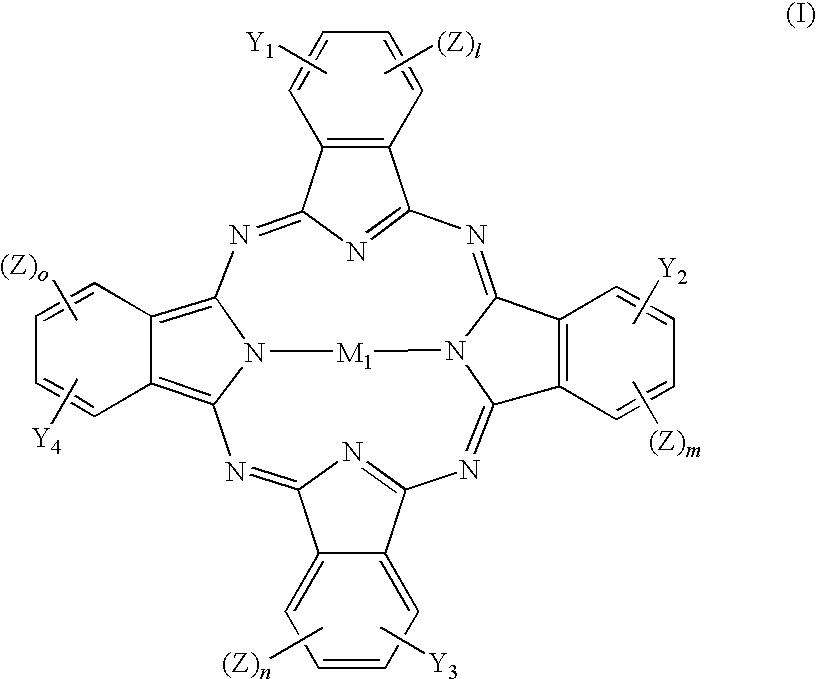
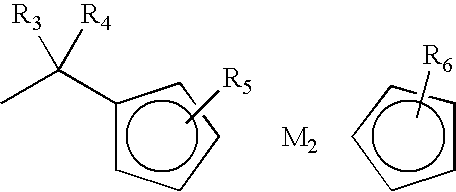
[0106] Tetra-(α-2,4-dimethyl-3-pentyloxy) copper phthalocyanine (CuPc, 30.0 g) was dissolved in 1,2-dichloroethane (300 mL) and stirred for 0.5 h in a dry flask. Chloromethylferrocene (FcCH2Cl, 12.2 g) (FcCH2Cl was prepared by the known method described in J. Am. Chem. Sodc 1966, 88, 3442-3444) was added, and subsequently aluminium chloride (1.56 g) was crushed and added promptly. The mixture was stirred for 10 min, warmed up to 80 AC for another 4 h and then cooled to room temperature.
[0107] The mixture aforementioned was poured into a solution mixed with ice (180 g) and water (180 mL) in a beaker (1 L), and stirred; the temperature was controlled about 10° C. for 1 h. The solution was poured into a separatory funnel and extracted with water (300 mL) twice. The organic layers were collected, concentrated to approximately 70 g, and poured into a methanol (900 mL) solution with stirring by a mechanical agitator at a temperature about 10° C. The precipitate was formed and filtered. T...
example 2
[0108] Tetra-(α-2,4-dimethyl-3-pentyloxy) copper phthalocyanine (CuPc, 30.0 g) was dissolved in 1,2-dichloroethane (300 mL) and stirred for 0.5 h in a d flask. Chloromethylferrocene (FcCH2Cl, 12.2 g) was added, and then sulfuric acid (98%, 0.86 g) was slowly added. The mixture was stirred for 10 min, warmed to 90° C. for another 3 h, and then cooled to room temperature.
[0109] The solution aforementioned was poured into a beaker equipped with a magnetic stirrer, and charged with a mixture of ice (60 g) and water (300 mL). The mixture was stirred and the temperature was controlled at 10˜15° C. for 0.5 h. The solution was poured into a separatory funnel, and extracted with water (300 mL) twice. The organic layers were collected, concentrated to approximately 70 g, and poured into a methanol (900 mL) solution with stirring by a mechanical agitator at a temperature about 10° C. A precipitate was formed and filtered. The filtered cake was washed with methanol (30 mL) three times to give ...
example 3
[0110] Tetra-(α-2,4-dimethyl-3-pentyloxy) copper phthalocyanine (CuPc, 30.0 g) was dissolved in 1,2-dichloroethane (300 mL) in a dry flask and stirred for 0.5 h. Chloromethylferrocene (FcCH2Cl, 12.2 g) was added, and subsequently aluminium chloride (2.33 g) was crushed and added promptly. The mixture was stirred for 10 min, warmed to 80° C. for another 6 h and then cooled to room temperature.
[0111] The solution aforementioned was poured into a beaker equipped with a magnetic stirrer, and charged with a mixture of ice (180 g) and water (180 mL). The mixture was stirred, and the temperature was controlled at 10° C. for 1 h The solution was poured into a separatory funnel, and extracted with water (300 mL) twice. The organic layers were collected, concentrated to approximately 100 g, and poured into a methanol (900 mL) solution with stirring by a mechanical agitator with the temperature controlled at 10° C. A precipitate was formed and filtered. The filtered cake was washed with metha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molar ratio | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com