Enzyme electrode
a technology of enzyme electrodes and electrodes, applied in the field of enzyme electrodes, can solve the problems of low reproducibility (not stabilized), inability to carry out stable operation, and limited operation conditions of enzyme electrodes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0056]In this example, the influence of the blending amount of the high-resistance particle to the resistance value of the enzyme electrode will be examined.
(Production of Enzyme Electrode)
[0057]In the production of the enzyme electrode, first, aluminum oxide (“AEROSIL”; manufactured by Degussa AG) and platinized carbon (“IFPC40-II”; manufactured by ISHIFUKU Metal Industry Co., Ltd.) were extracted so that the addition rates thereof are respectively changed and the total amount became 60 mg and were fully mixed to produce a mixed powder. Next, 1000 μL of a GDH solution of 1250 U / mL was added into the mixed powder, and they were mixed to prepare a mixed solution. The mixed solution was left at rest for 6 hours to preliminarily adsorb GDH on platinized carbon, and centrifuged to remove a supernatant fluid. A slurry after the supernatant fluid was removed was powdered by vacuum drying, and 100 μL of liquid paraffin was added into the powder. They were mixed well to produce a paste. Thi...
example 2
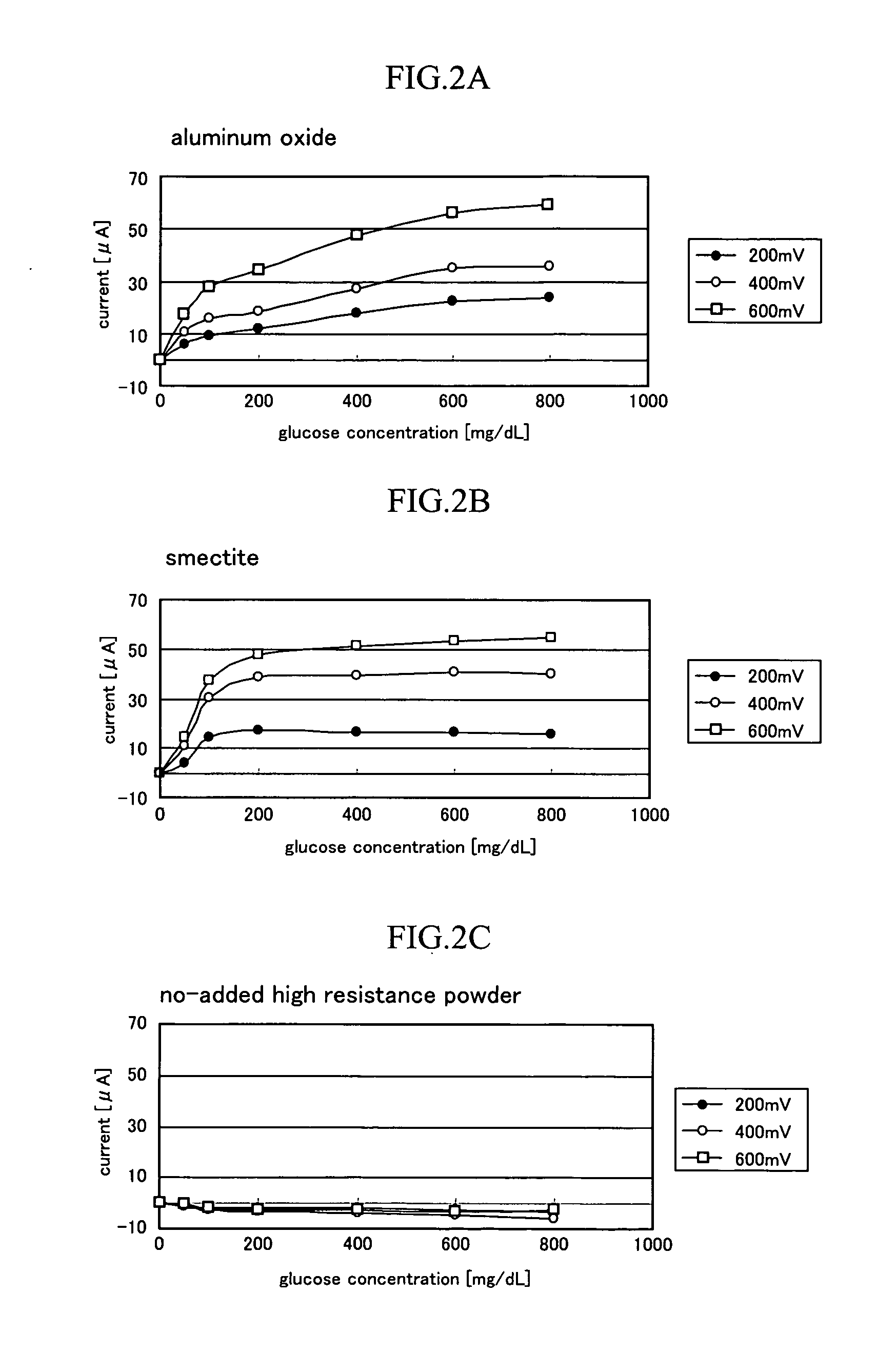
[0059]In the example, electric generating capacity as a glucose fuel cell was evaluated for a GDH platinized carbon enzyme electrode.
[0060]The electric generating capacity was evaluated by immersing an enzyme electrode as an anode electrode, and a platinum electrode as a cathode electrode into an electrolyte solution to form a battery. The electric generating capacity was measured in an air release atmosphere with a temperature of the battery set to 25° C.
[0061]Four kinds (one kind is aluminum oxide free) of enzyme electrodes having a different blending ratio of platinized carbon and aluminum oxide were produced as in example 1. The enzyme electrode has a diameter of 3 φ and a thickness of 2 mm. As the platinum electrode, a wire platinum electrode having a diameter of 1 φ and a length of 5 cm was used.
[0062]An electrolyte capacity was set to 10 mL using a phosphoric acid buffer (pH: 6.0) of 100 mmol / L as the electrolyte solution. Glucose was dissolved in the electrolyte solution so ...
example 3
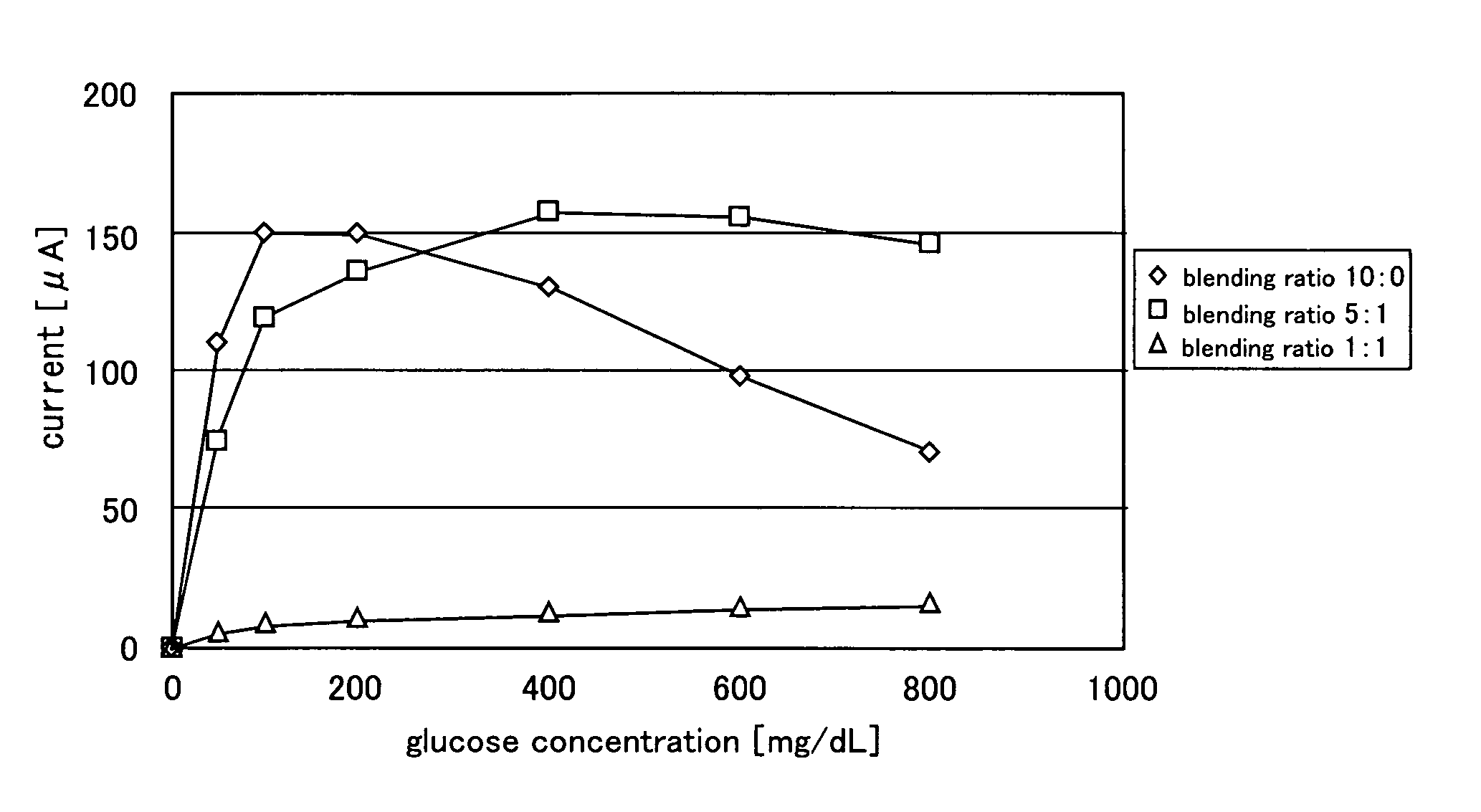
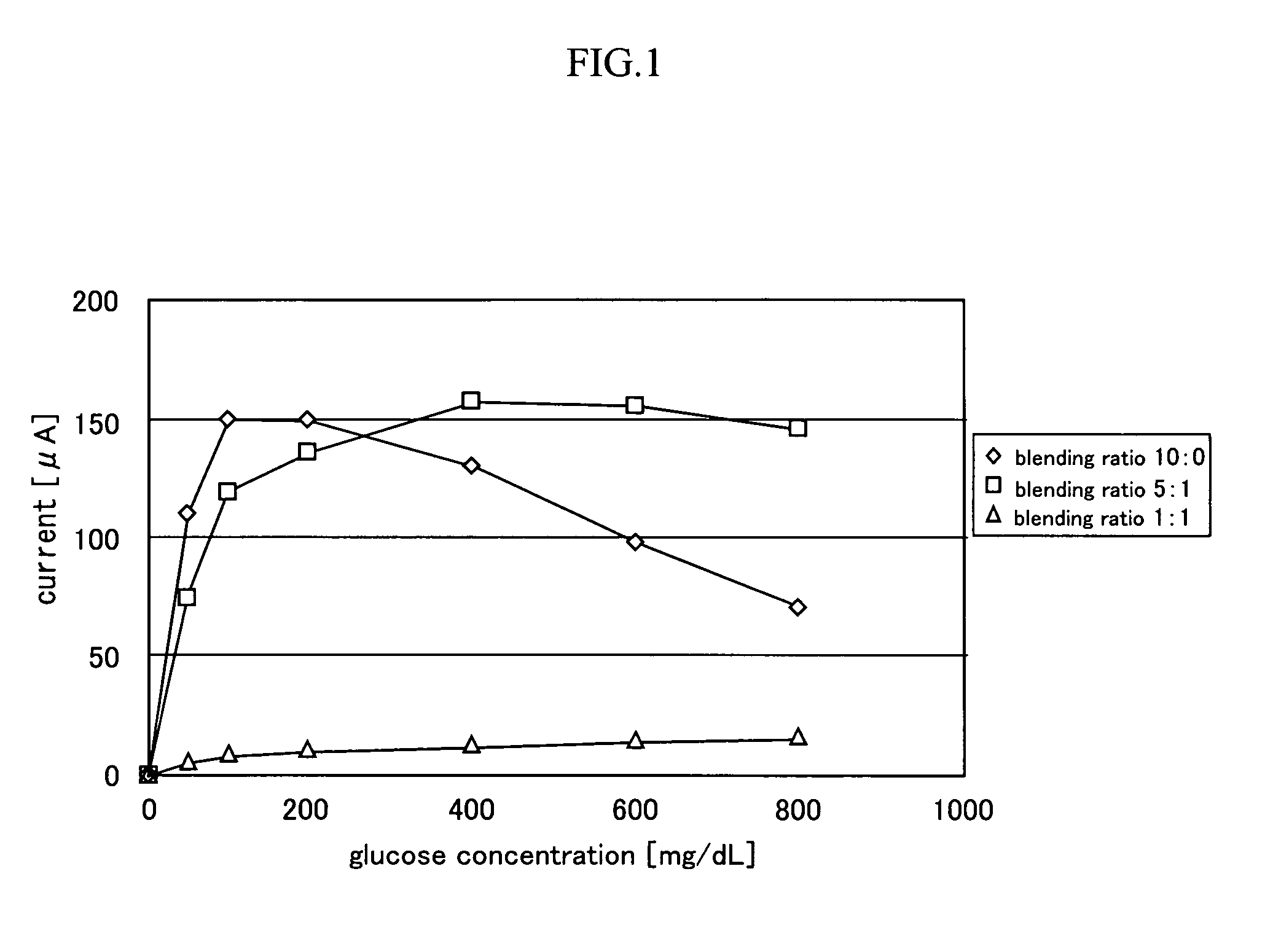
[0066]In this example, the dependency of the glucose concentration of the measured current value for each of the enzyme electrode into which aluminum oxide was added and the enzyme electrode into which aluminum oxide was not added was evaluated.
[0067]The glucose concentration was adjusted by calculating so that the glucose concentration became the aimed concentration to the electrolyte solution used in example 2, and dissolving glucose. The aimed concentration was set to 0 mg / dL, 50 mg / dL, 100 mg / dL, 200 mg / dL, 400 mg / dL, 600 mg / dL and 800 mg / dL.
[0068]A current value was measured as a response value when applying voltage between the enzyme electrode and the platinum electrode by a linear sweep voltammetrical method.
[0069]Three kinds of enzyme electrodes (one kind is aluminum oxide free) having a different blending ratio of the platinized carbon and aluminum oxide were produced as in example 1. Both the enzyme electrode and the platinum electrode had a diameter of 3 φ and a thickness...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com