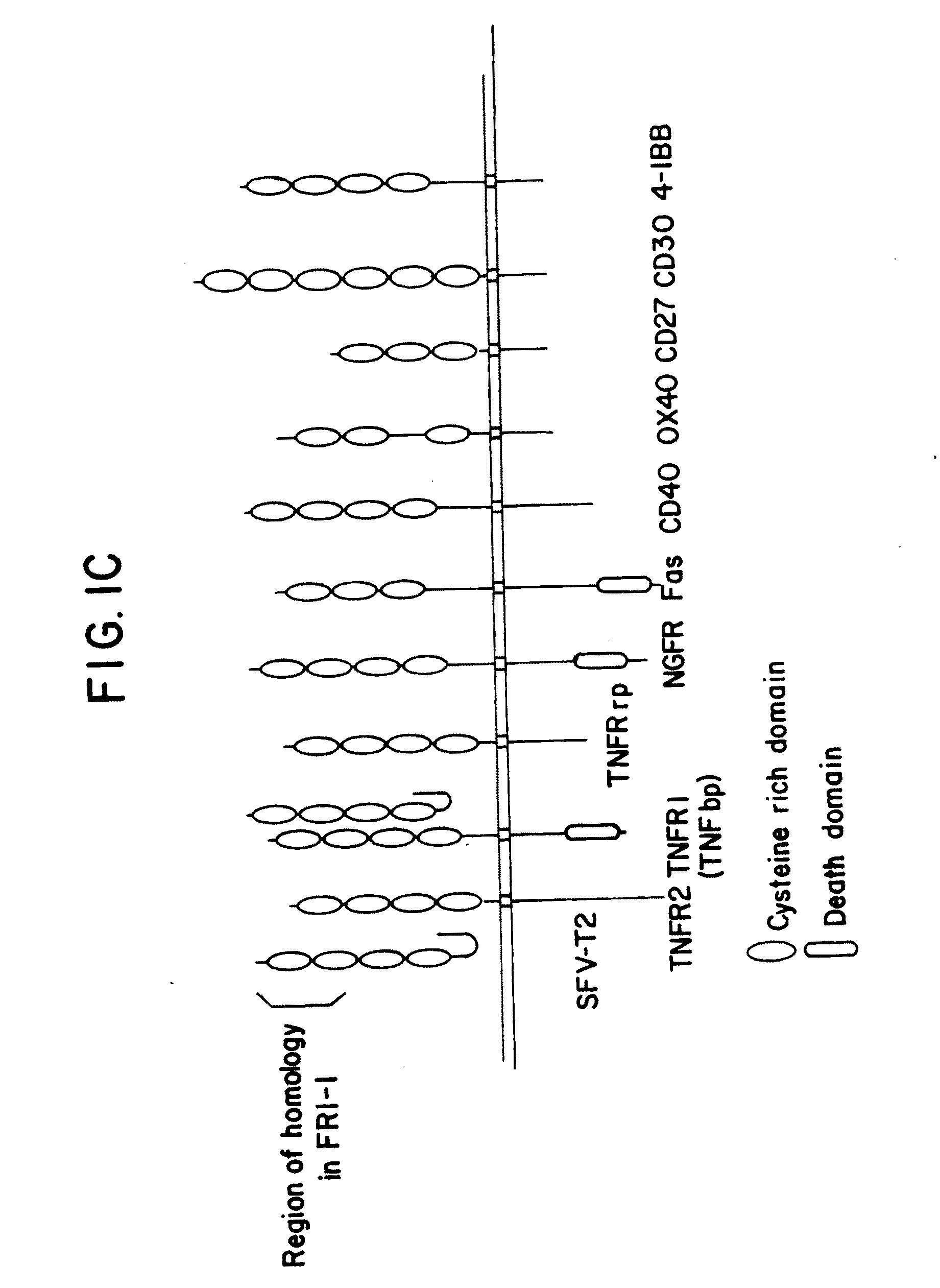
Osteoprotegerin
a technology of osteoprotegerin and polypeptide, which is applied in the direction of peptide/protein ingredients, instruments, drug compositions, etc., can solve the problems of systemic delivery of tnf inducing toxic shock and widespread tissue necrosis, and achieves the effects of promoting bone accumulation, preventing bone resorption, and increasing bone density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Identification and Isolation of the Rat OPG cDNA
[0106]Materials and methods for cDNA cloning and analysis are described in Maniatis et al, ibid. Polymerase chain reactions (PCR) were performed using a Perkin-Elmer 9600 thermocycler using PCR reaction mixture (Boehringer-Mannheim) and primer concentrations specified by the manufacturer. In general, 25-50 μl reactions were denatured at 94° C., followed by 20-40 cycles of 94° C. for 5 seconds, 50-60° C. for 5 seconds, and 72° C. for 3-5 minutes. Reactions were the treated for 72° C. for 3-5 minutes. Reactions were then analyzed by gel electrophoresis as described in Maniatis et al., ibid.
[0107]A cDNA library was constructed using mRNA isolated from embryonic d20 intestine for EST analysis (Adams et al. Science 252, 1651-1656 (1991)). Rat embryos were dissected, and the entire developing small and large intestine removed and washed in PBS. Total cell RNA was purified by acid guanidinium thiocyanate-phenol-chloroform extraction (Chomczyn...
example 2
OPG mRNA Expression Patterns in Tissues
[0118]Multiple human tissue northern blots (Clonetech) were probed with a 32P-dCTP labelled FRI-1 PCR product to detect the size of the human transcript and to determine patterns of expression. Northern blots were prehybridized in 5×SSPE, 50% formamide, 5× Denhardt's solution, 0.5% SDS, and 100 μg / ml denatured salmon sperm DNA for 2-4 hr at 42° C. The blots were then hybridized in 5×SSPE, 50% formamide, 2× Denhardt's solution, 0.1% SDS, 100 μg / ml denatured salmon sperm DNA, and 5 ng / ml labelled probe for 18-24 hr at 42° C. The blots were then washed in 2×SSC for 10 min at RT, 1×SSC for 10 min at 50° C., then in 0.5×SSC for 10-15 min.
[0119]Using a probe derived from the rat gene, a predominant mRNA species with a relative molecular mass of about 2.4 kb is detected in several tissues, including kidney, liver, placenta, and heart. Highest levels are detected in the kidney. A large mRNA species of Mr 4.5 and 7.5 kb was detected in skeletal muscle a...
example 3
Systemic Delivery of OPG in Transgenic Mice
[0120]The rat OPG clone pB1.1 was used as template to PCR amplify the coding region for subcloning into an ApoE-liver specific expression vector (Simonet et al. J. Clin. Invest. 94, 1310-1319 (1994), and PCT Application No. US94 / 11675 and co-owned U.S. Ser. No. 08 / 221,767. The following 5′ and 3′ oligonucleotide primers were used for PCR amplification, respectively:
(SEQ ID NO: 9)5′-GACTAGTCCCACAATGAACAAGTGGCTGTG-3′(SEQ ID NO: 10)5′-ATAAGAATGCGGCCGCTAAACTATGAAACAGCCCAGTGACCATTC-3′
[0121]The PCR reaction mixture (Boehringer-Mannheim) was treated as follows: 94° C. for 1 minute, 1 cycle; 94° C. for 20 sec, 62° C. for 30 sec, and 74 C for 1 minute, 25 cycles. Following amplification, the samples were purified over Qiagen PCR columns and digested overnight with SpeI and NotI restriction enzymes. The digested products were extracted and precipitated and subcloned into the ApoE promoter expression vector. Prior to microinjecting the resulting clone...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com