Process for Converting Alkaline-Earth Metal Chlorides to Tungstates and Molybdates and Applications Thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
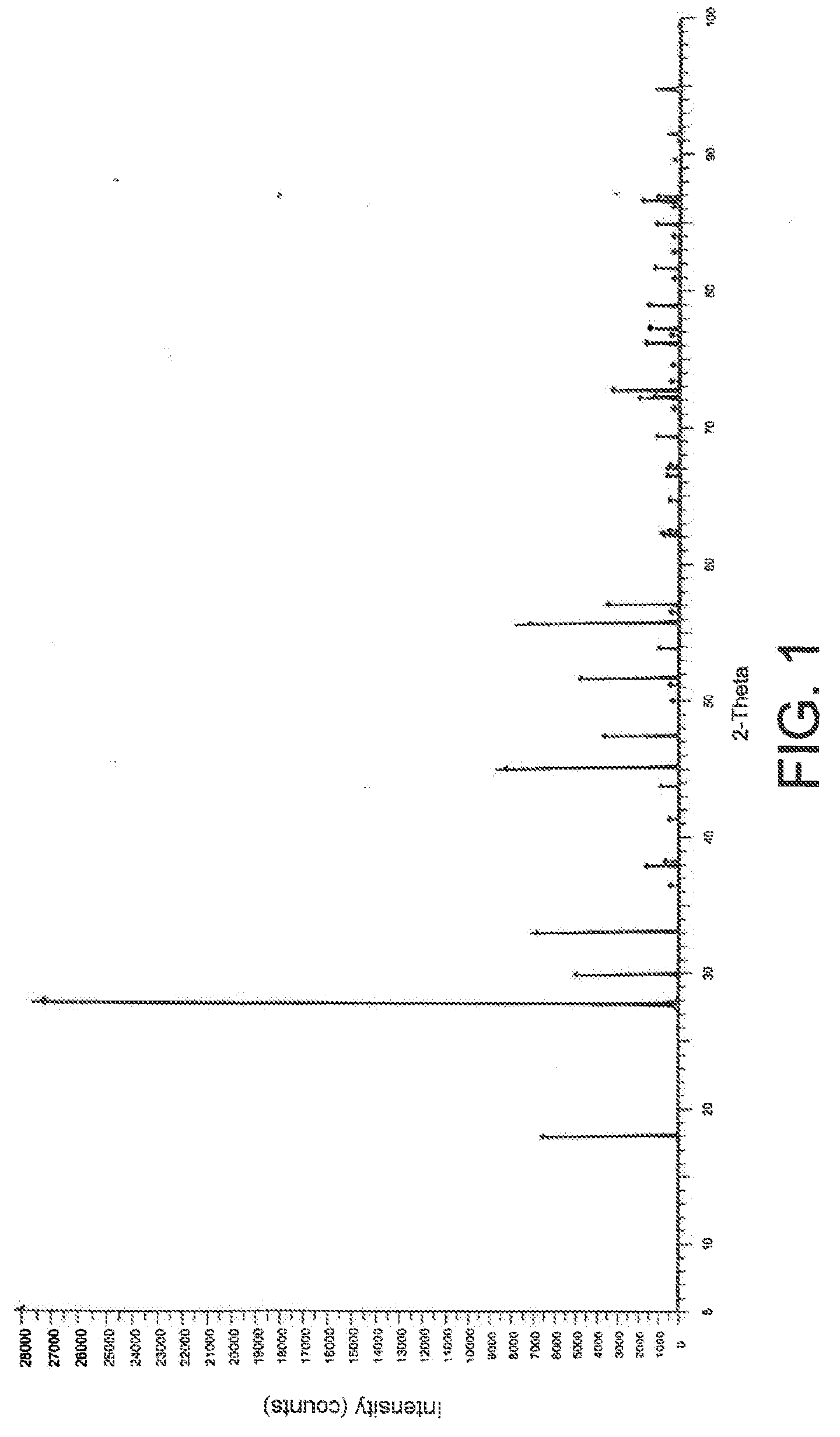
Conversion of SrCl2 to SrWO4 in the Absence of any Other Chloride
[0064]In an alumina crucible, the following were mixed at ambient temperature (20-25° C.): 1 g of SrCl2, having a purity greater than 99%, and 9 g of an LiCl / KCl eutectic, formed from 4.005 g of LiCl and from 4.995 g of KCl; then, still at ambient temperature, 2.0567 g of K2WO4 were added to the mixture thus obtained.
[0065]The crucible was introduced into a quartz tube that was sealed and that was heated at 500° C. at a rate of around 5° C. per minute. The tube was kept at 500° C. for 5 hours, then it was cooled at a rate of around 2° C. per minute until a temperature of 300° C. was reached. The contents of the crucible was then quenched in air.
[0066]A solid was thus recovered, which was submerged in ultrapure water at high temperature (25° C. or 100° C.) in order to make the SrCl2 that had not reacted pass into solution, then the assembly was filtered over a Büchner funnel, using a filter with a cut-off threshold of 0...
example 2
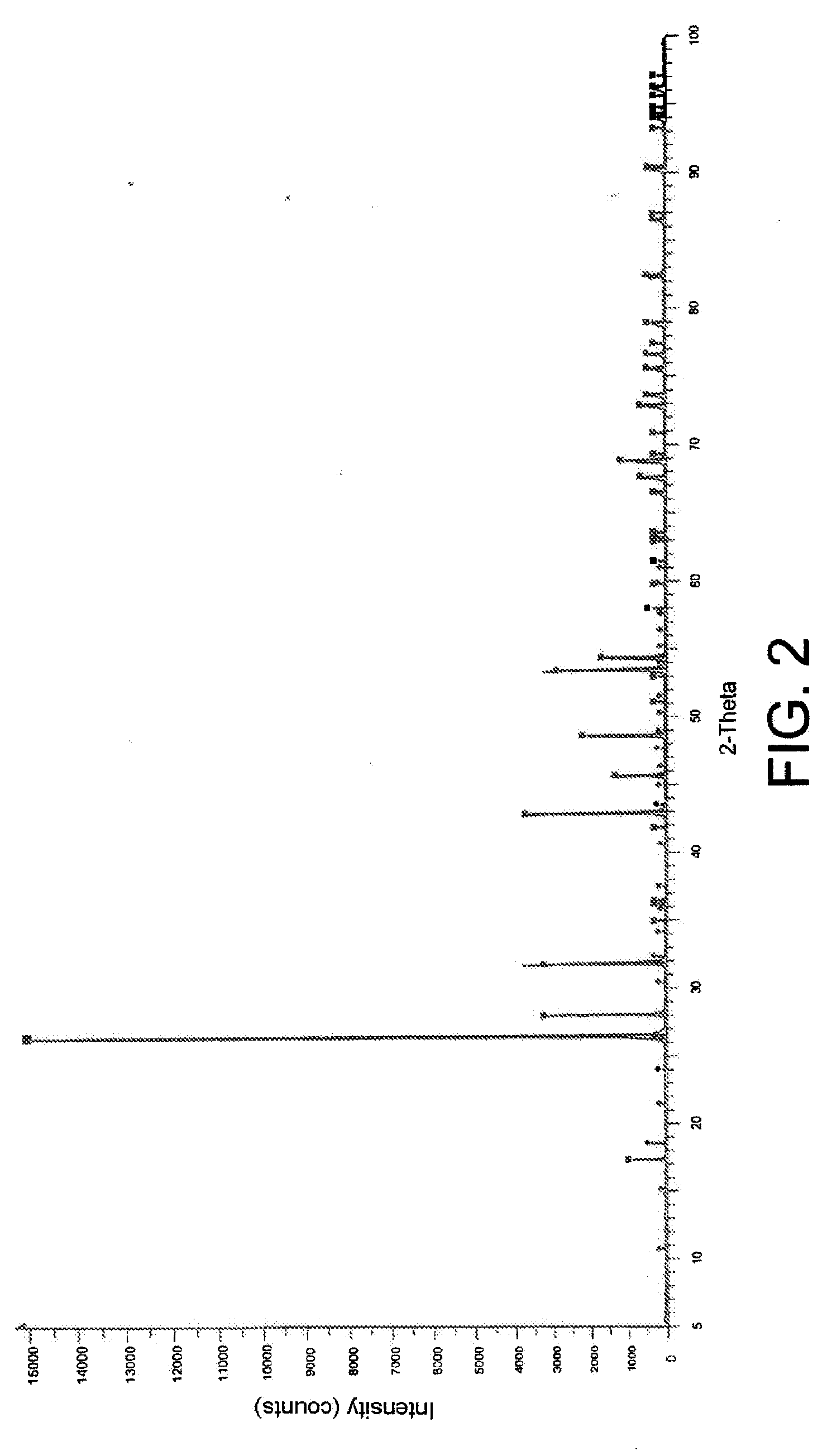
Conversion of BaCl2 to BaWO4 in the Absence of any Other Chloride
[0069]The same procedure as that described in Example 1 above was followed, except that 1 g of BaCl2 (instead of the gram of SrCl2) was dissolved in the eutectic and that 1.5657 g of K2WO4 was used.
[0070]The X-ray diffraction pattern of the powder thus obtained is represented in FIG. 2. This diffraction pattern shows a good agreement between the peaks recorded and those of the card calculated for BaWO4 (00-043-0646 (*)), proving that the BaCl2 initially mixed with the eutectic solvent was indeed converted to BaWO4.
[0071]The yield of the reaction, determined by weighing the BaWO4 and comparing the result of the weighing with the theoretical mass corresponding to 100% reaction, is greater than 90%.
example 3
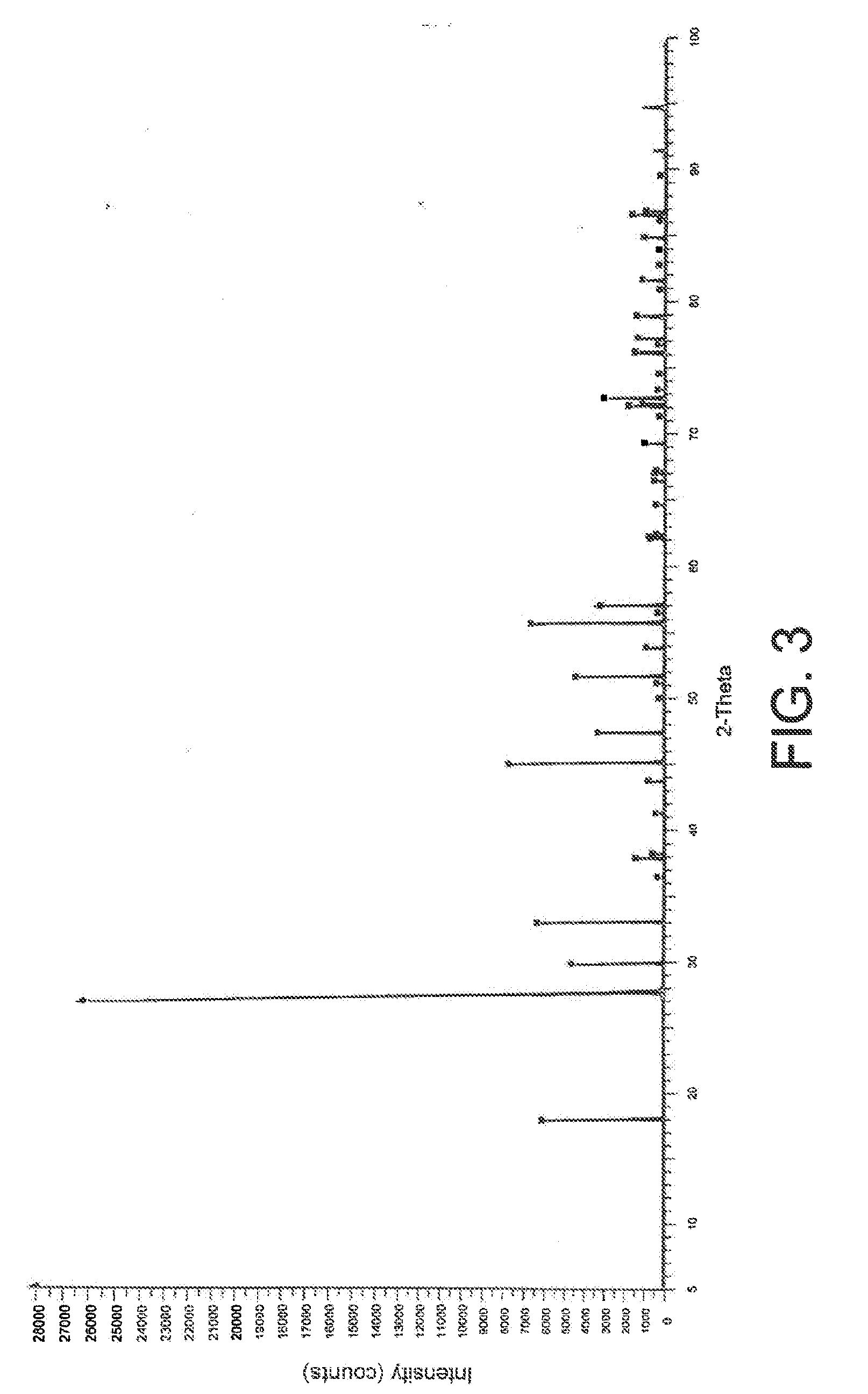
Conversion of SrCl2 to SrWO4 in the Presence of RbCl and CsCl
[0072]The same procedure as that described in Example 1 above was followed except that 1 g of a mixture of SrCl2, RbCl and CsCl (1 / 0.5 / 0.5 w / w) was dissolved in the eutectic instead of the gram of SrCl2.
[0073]The X-ray diffraction pattern of the powder thus obtained is represented in FIG. 3.
[0074]This diffraction pattern is almost identical to that represented in FIG. 1, which proves, on the one hand, that only the SrCl2 was converted to tungstate and, on the other hand, that the presence of the two alkali metal chlorides in the reaction medium had no influence on this conversion.
[0075]The yield of the reaction (determined as in Example 1) is greater than 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com