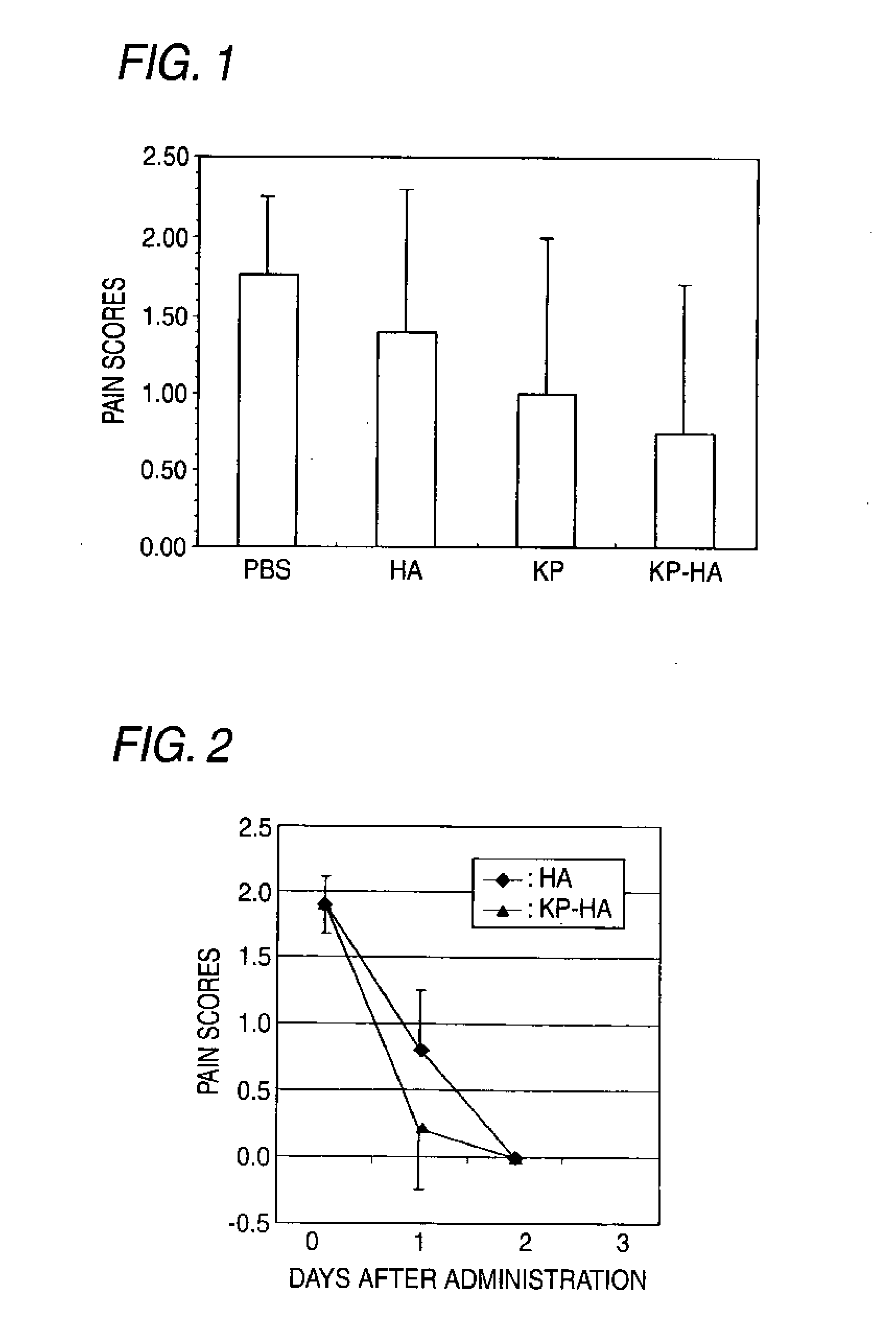
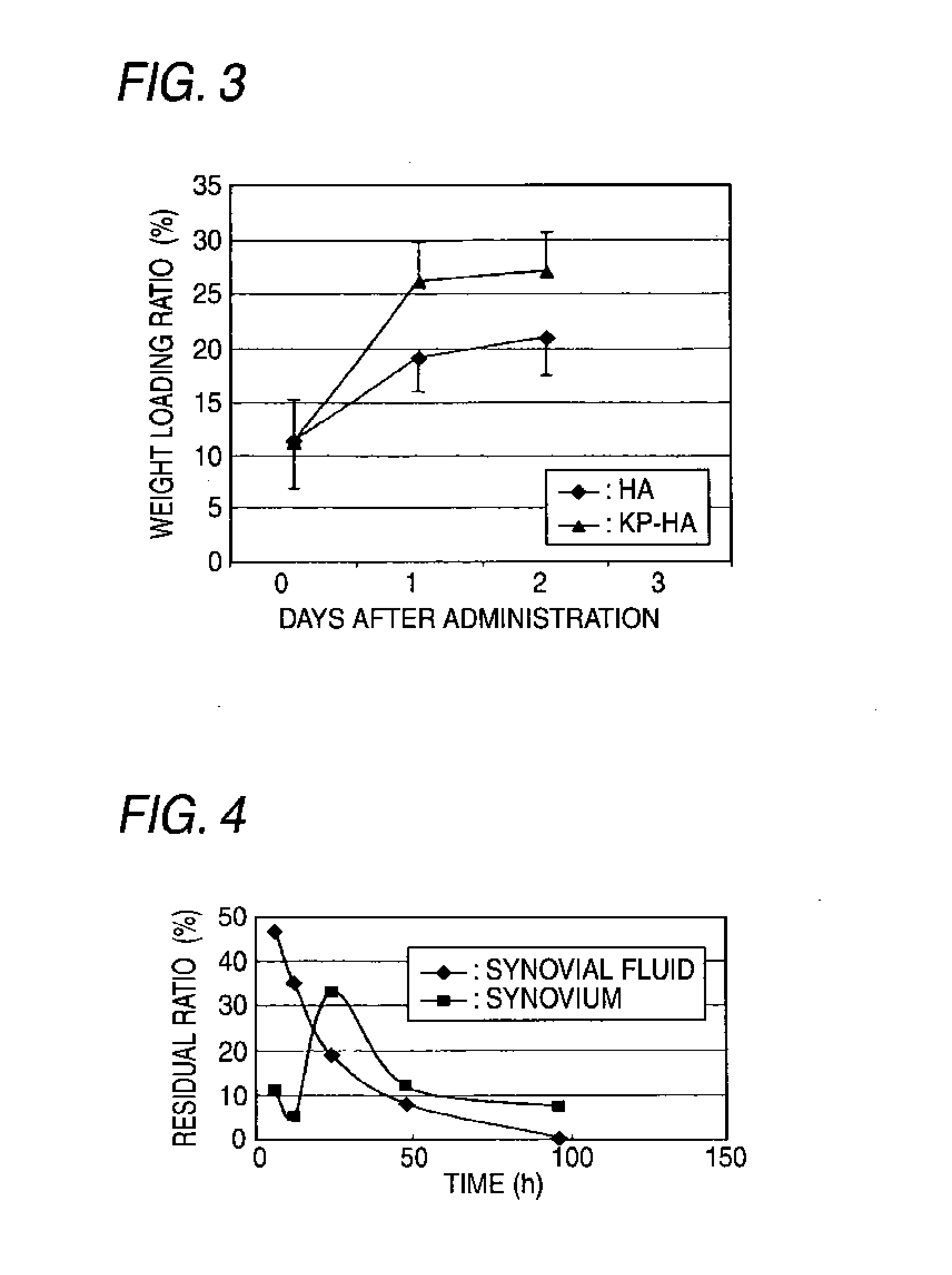
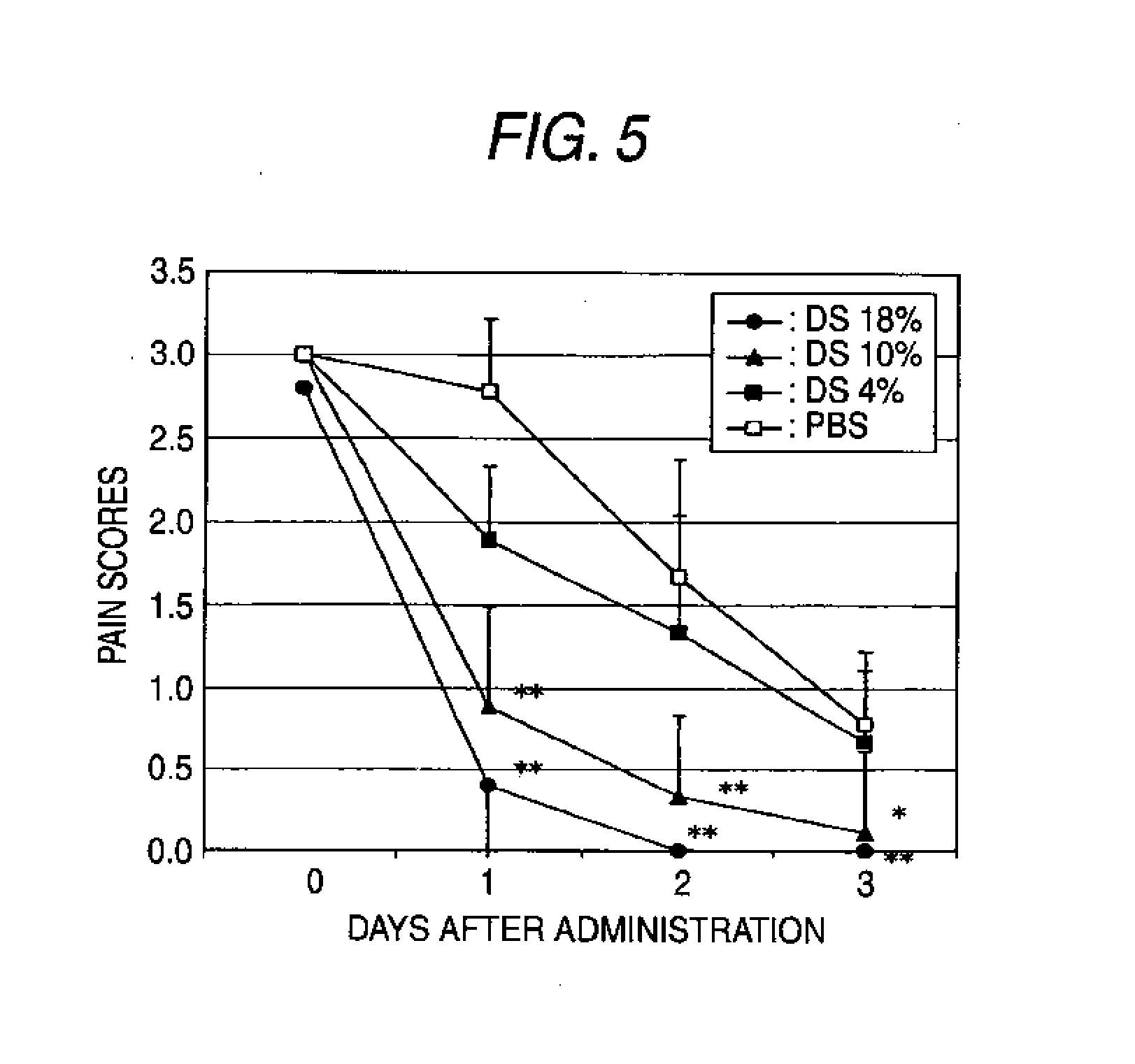
Hyaluronic acid derivative and drug containing the same
a technology of hyaluronic acid and derivatives, which is applied in the direction of biocide, drug composition, transportation and packaging, etc., can solve the problems of short time period for continuing the effect of nsaids and the method not becoming a basic remedy for arthritis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0146]The present invention is described below more specifically based on Examples. However, there is no intention to limit the technical scope of the present invention by this.
[0147]In this connection, all of the hyaluronic acid and sodium hyaluronate used in the following Examples were purchased from Seikagaku Corporation.
[0148]Hereinafter, as the phosphate buffered saline (PBS), 5 mM PBS was used in the following Examples, unless otherwise indicated.
production examples
Reference Example 1
Synthesis of t-butoxycarbonyl-aminopropanol (Boc-NH(CH2)3OH) (Boc-aminopropanol)
[0150]In 10 ml of dichloromethane, 1.542 g (20.5 mmol) of aminopropanol was dissolved, and 10 ml of a 4.484 g (20.5 mmol) di-t-butyl dicarbonate (Boc2O) / dichloromethane solution was slowly added dropwise thereto under ice-cooling. Thereafter, the reaction solution was returned to room temperature and stirred for 2 hours and 40 minutes, disappearance of the starting materials was confirmed by thin layer chromatography (TLC), and then dichloromethane was evaporated under reduced pressure. The reaction quantitatively progressed, and an oily substance was obtained at a yield of 3.92 g. The structure was identified by 1H-NMR (CDCl3).
[0151]1H-NMR (500 MHz, CDCl3) δ (ppm)=1.46 (9H, s, Boc), 1.66 (2H, quant, —NHCH2CH2CH2O—), 3.27 (3H, m, —NHCH2CH2CH2O—), 3.66 (2H, m, —NHCH2CH2CH2O—), 4.91 (1H, br, CH2OH)
example 1
Synthesis of Aminopropanol-Ketoprofen Hydrochloride
1) Synthesis of Boc-Aminopropanol-Ketoprofen
[0152]In 14 ml of dichloromethane, 2.371 g (13.5 mmol) of Boc-aminopropanol and 3.441 g (13.5 mmol) of ketoprofen (manufactured by Tokyo Kasei Kogyo) were dissolved, and 323 mg (2.6 mmol) of 4-dimethylaminopyridine (DMAP) and 2.833 g (14.8 mmol) of water-soluble carbodiimide hydrochloride (WSCI.HCl) / 14 ml dichloromethane were added thereto in this order under ice-cooling. After returning to room temperature and stirring overnight, dichloromethane was evaporated under reduced pressure, and ethyl acetate was added thereto, followed by separation by washing with 5% citric acid twice, water, 5% sodium hydrogen carbonate twice, water and saturatedbrine consecutively. After dehydration drying with sodium sulfate, ethyl acetate was evaporated under reduced pressure to give 5.430 g of the titled compound (yield 98%). The structure was identified by 1H-NMR (CDCl3).
[0153]1H-NMR (500 MHz, CDCl3) δ (p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com