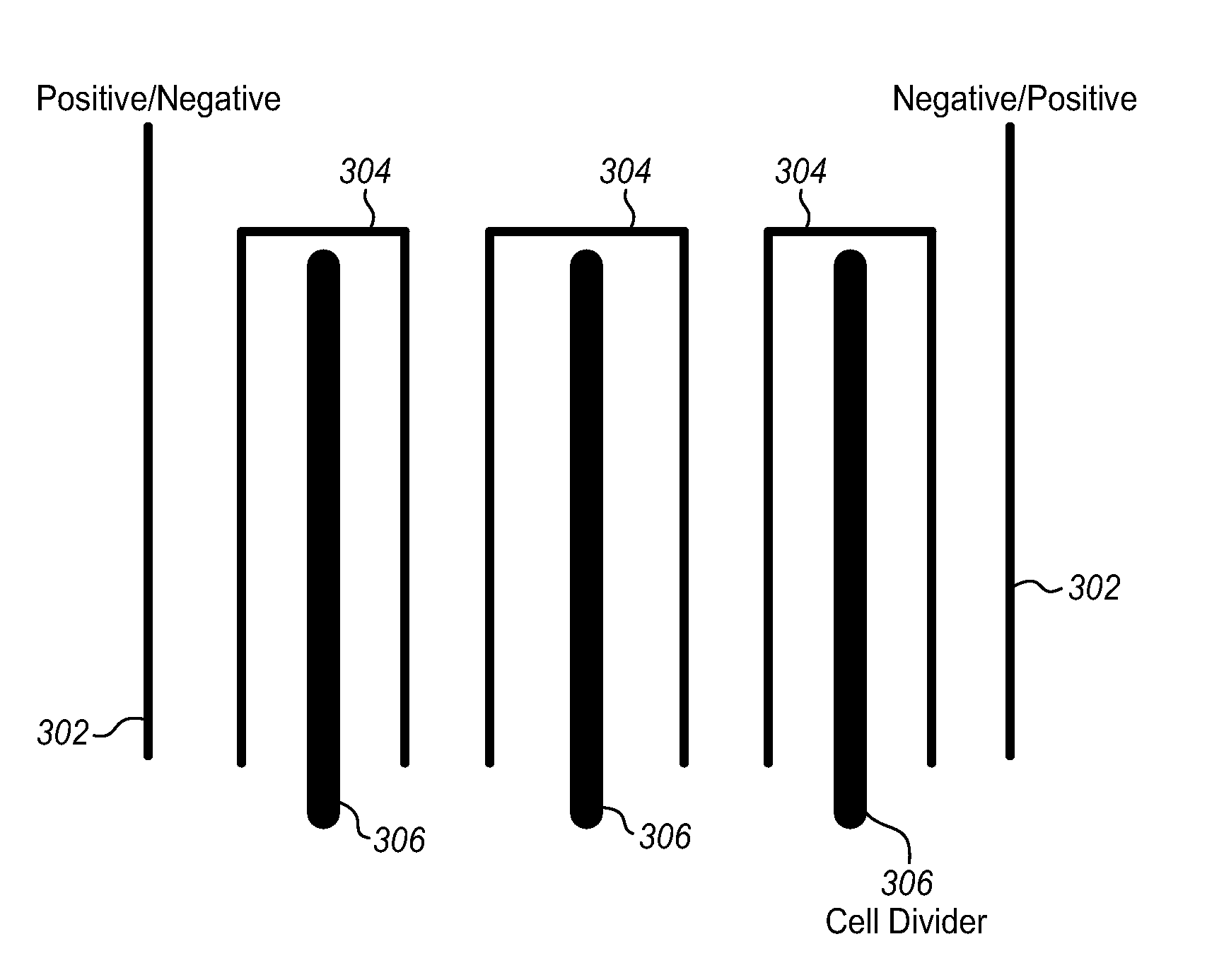
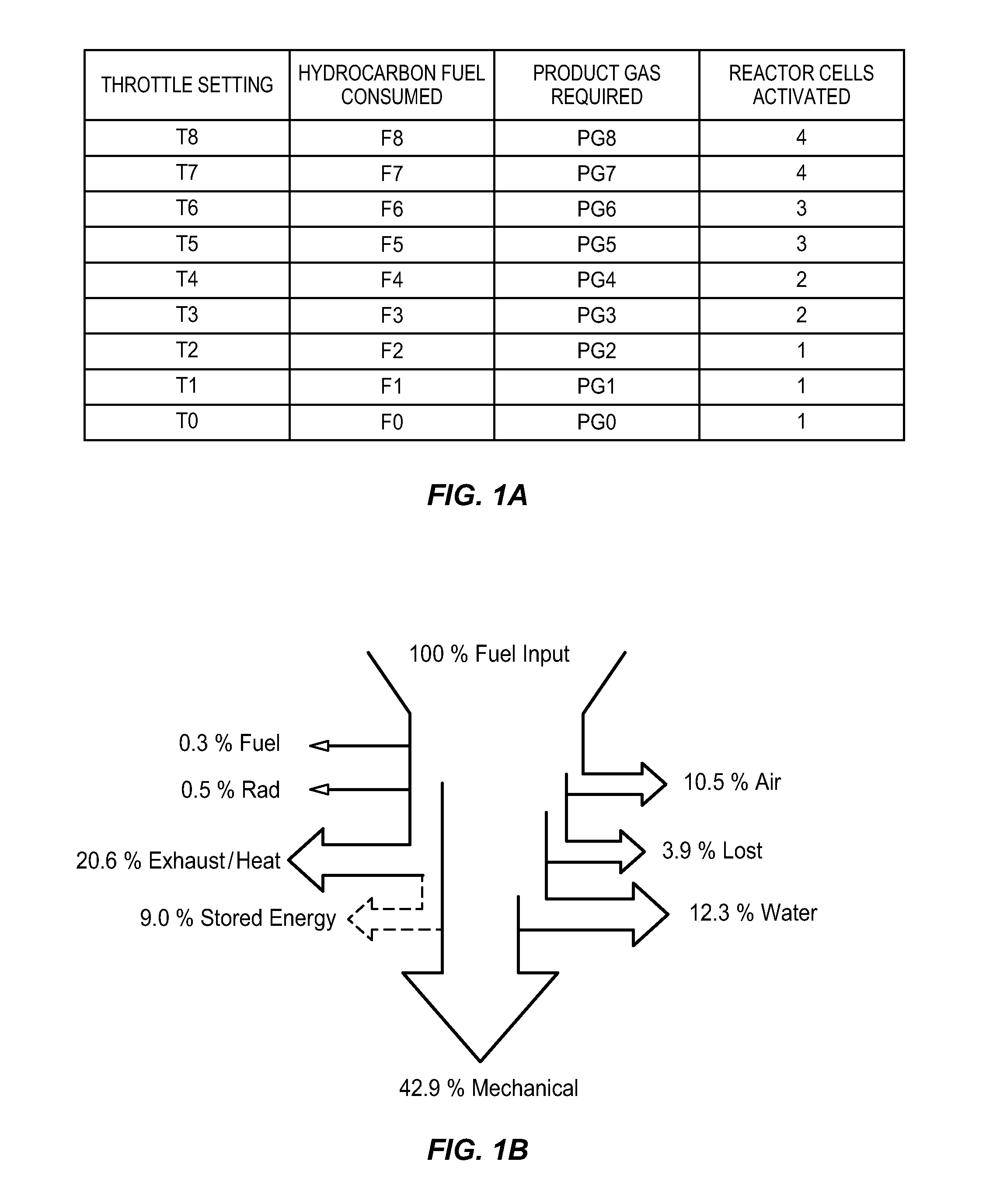
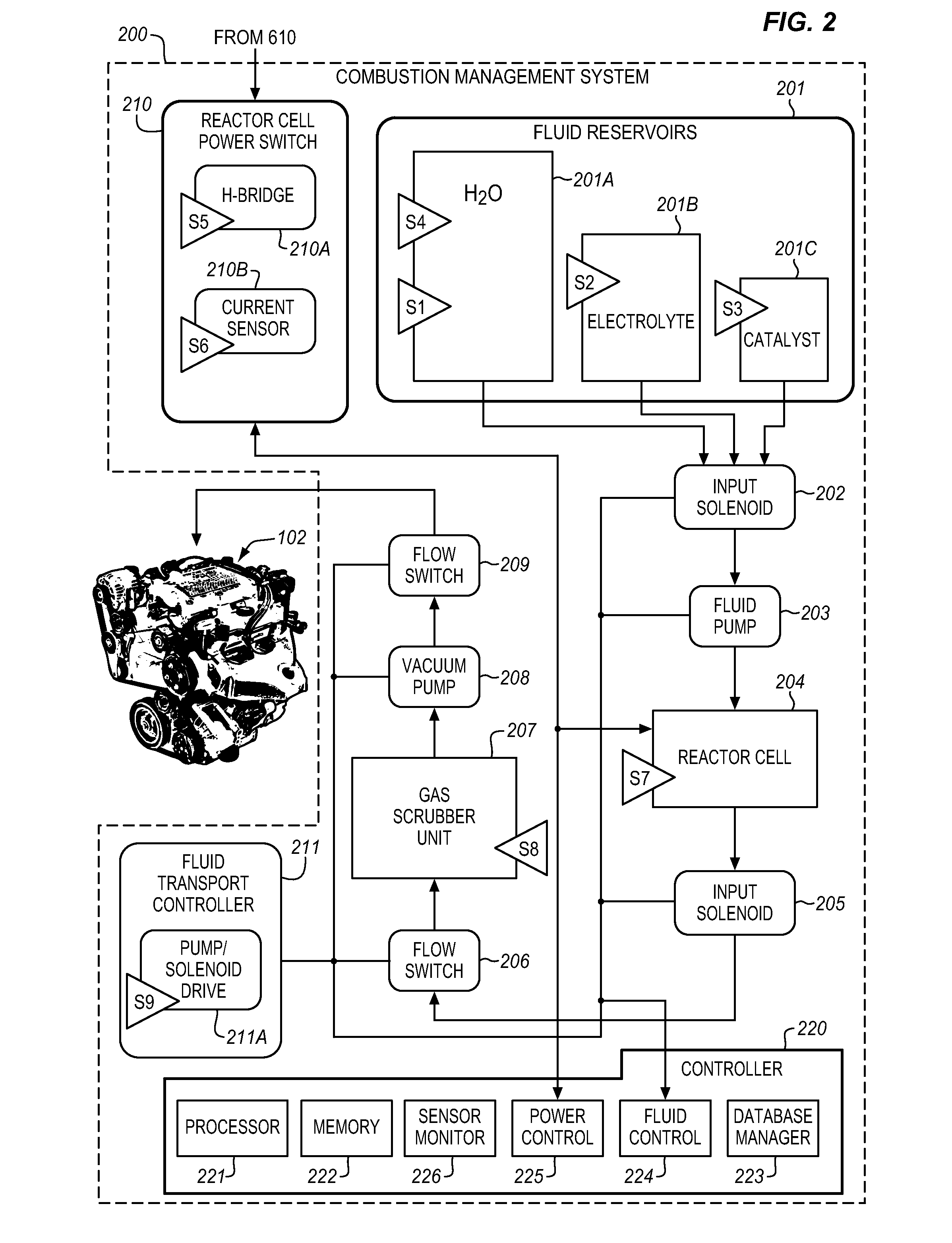
[0021]The present
Product Gas Generator For Producing A Substantially Stoichiometric Mix Of
Hydrogen And
Oxygen (termed “Product
Gas Generator” herein) works in conjunction with a Combustion
Management System, which models each
hydrocarbon combustion application, and the Product
Gas Generator supplies a
product gas, comprising a dynamic mixture of nascent
hydrogen (H) and
oxygen (O), to the
internal combustion engine to propagate the formation of
hydroxide radicals (OH) and thereby to improve the level of completion of the hydrocarbon
combustion reaction. Atomic
hydrogen (or nascent hydrogen) is the species denoted by H (atomic), contrasted with di-hydrogen, the usual “hydrogen” (H2) commonly involved in chemical reactions. Being monatomic, nascent hydrogen (H) atoms are much more reactive and, thus, a much more effective
reducing agent than ordinary diatomic H2 atoms. The Combustion Management System provides product gas volumetric requirement information and takes into account the engine style, primary torque requests, and hydrocarbon fuel consumption information to develop an
operating system specific application that produces consistent measurable results. Stoichiometric models are used versus
trial and error data obtained from running the engine on a dynomometer through various load and engine speed conditions, which saves time and money while insuring that each Combustion Management System application is adequate for its intended use.
[0028]Unlike the HRM, which introduces a competitive reaction into the
internal combustion engine, this approach directly addresses the
primary reaction driving the hydrocarbon combustion mechanism toward completion. This approach creates a twofold increase in the reactive tendency toward completion.
Hydroxide radicals (OH) are lighter than the standard diatomic
oxygen (O2) being administered, which allows for greater diffusivity and an increased potential for oxidative continuance to supersede
polymerization. Also, the higher oxidative potential of the
hydroxide radicals (OH) allow for
carbon chain cleaving reactions, thus creating more reactive sites on the hydrocarbon molecules and greater reaction distribution.
[0029]Fuel savings are achieved as a result of extracting more
stored energy from each hydrocarbon molecule. Every carbon-carbon and carbon-
hydrogen bond in the cylinders of the internal combustion engine represents
stored energy that could be translated into mechanical work. By promoting a higher degree of oxidative completion, the Combustion Management System extracts more energy from the hydrocarbon fuel. Similarly, emissions of particulate matter, hydrocarbons, and
carbon monoxide from the internal combustion engine are a direct result of this hydrocarbon combustion not propagating to completion. Therefore, furthering the combustive process has a direct and measurable
impact on both fuel consumption and emissions reduction.
[0030]As noted above,
polymerization is a problem in combustive reactions; and the primary causes of
polymerization within an engine cylinder are fuel
droplet size, turbulence, air composition, molecule size, and
reaction mechanism. The Combustion Management System addresses each of these dynamics to ensure successful and consistent reductions. The product gas
injection port not only administers the activated gas but also is designed to increase turbulence and ensure homogeneous mixing. Fuel injectors are modified or replaced to optimize
droplet size and injection timing. The activated
reaction mechanism generates more molecules of smaller size and greater separation. All of these factors combine to facilitate a near total reduction of particulate matter emissions.
[0031]The Combustion Management System is geared toward increasing the reactivity of the hydrocarbon fuel itself. By increasing the number of
active carbon sites in the fuel, which is present in the cylinders of the internal combustion engine, the statistical probability of oxygen reacting in the desired fashion is dramatically improved. Also, the addition of nascent hydrogen (H) in stoichiometric balance with oxygen (O) nullifies the competition between the hydrogen (H) and carbon (C) for oxidation. Also, the creation of more
active carbon sites reduces
residence time of
active oxygen and decreases the statistical probability that
nitrogen and oxygen will collide during the optimum temperature threshold.
Reaction rate reductions also serve to limit the timeframe where
NOx formation is energetically feasible.
 Login to View More
Login to View More