Polyelectrolyte Complexes
a polyelectrolyte complex and polyelectrolyte technology, applied in the field of associative polyelectrolyte complexes in water compositions, can solve the problems of unsightly surfaces requiring frequent cleaning and reapplication, and consumers are dissatisfied with their ability to prevent water and soil
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Small Scale Preparation of PAA and Chitosan Stock Solutions
[0060]A series of PAA / Chitosan PECs was prepared at several R values via gentle mixing using a magnetic stir bar for 1-2 minutes while stirring the solution, the minor polymeric component being placed in the vessel first as designated in the Table 1A with “1” followed by the major polymeric component added to the minor component solution designated with a “2” next to the weight used. The orders of addition change, of course, depending on the desired R value. The resulting associative PEC solutions were allowed to stir overnight and yielded clear solutions in all cases.
TABLE 1ACompositions of Chitosan / PAA PECsTotal20 wt %concen-CitrictrationStock AStock BAcidchargedFormulation(mL)(mL)(mL)H2Ogroups#R (a)(b)(c)(d)(mL)(mM)SCPAA10.250.4515 (1)0.7615 (2)0.552416.75801.29SCPAA20.510.7505 (1)0.6319 (2)0.497216.44921.30SCPAA30.770.9731 (1)0.5419 (2)0.437016.57101.29SCPAA41.051.1382 (1)0.4661 (2)0.396716.53421.28SCPAA51.271.2580 (2)0....
example 2
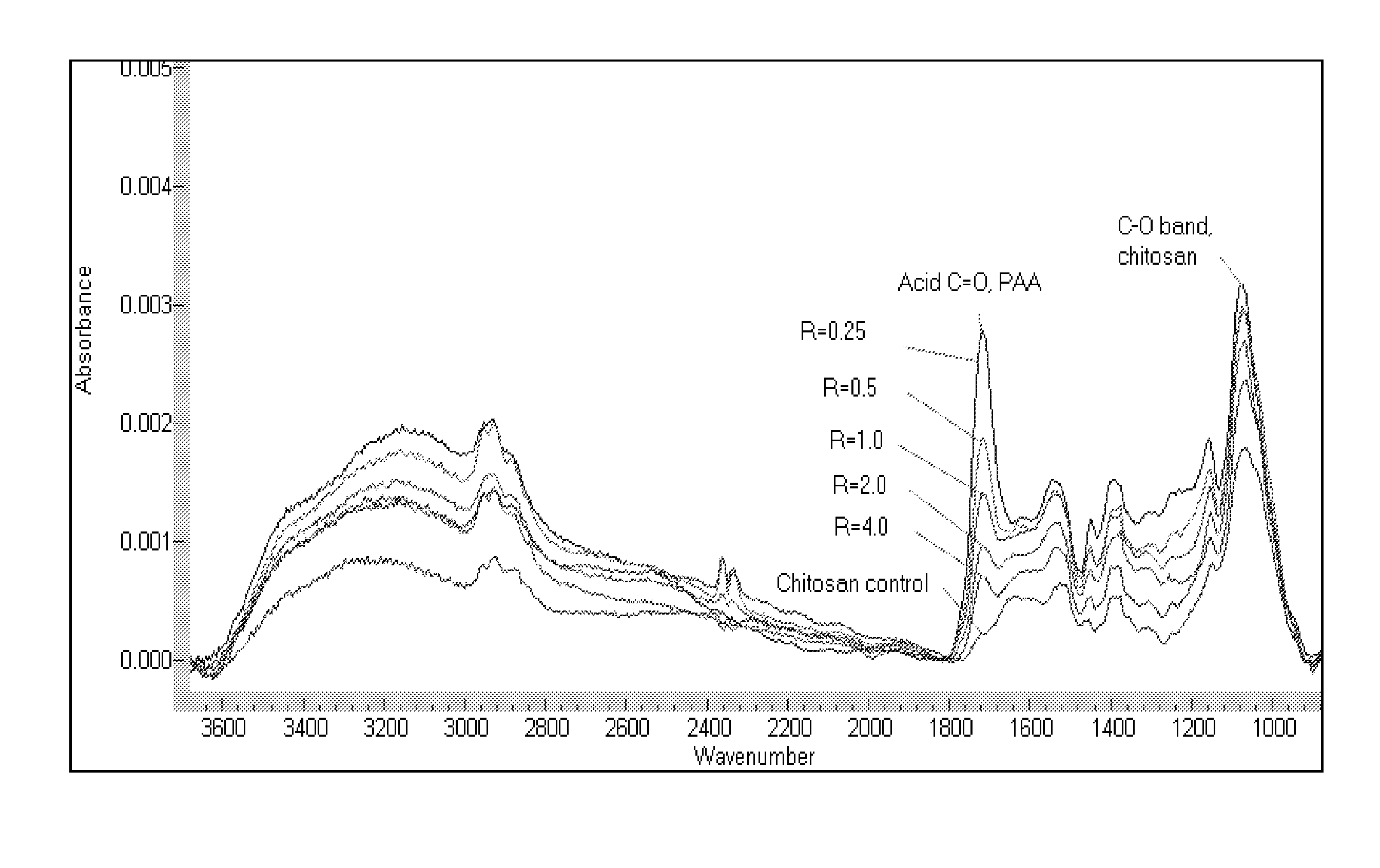
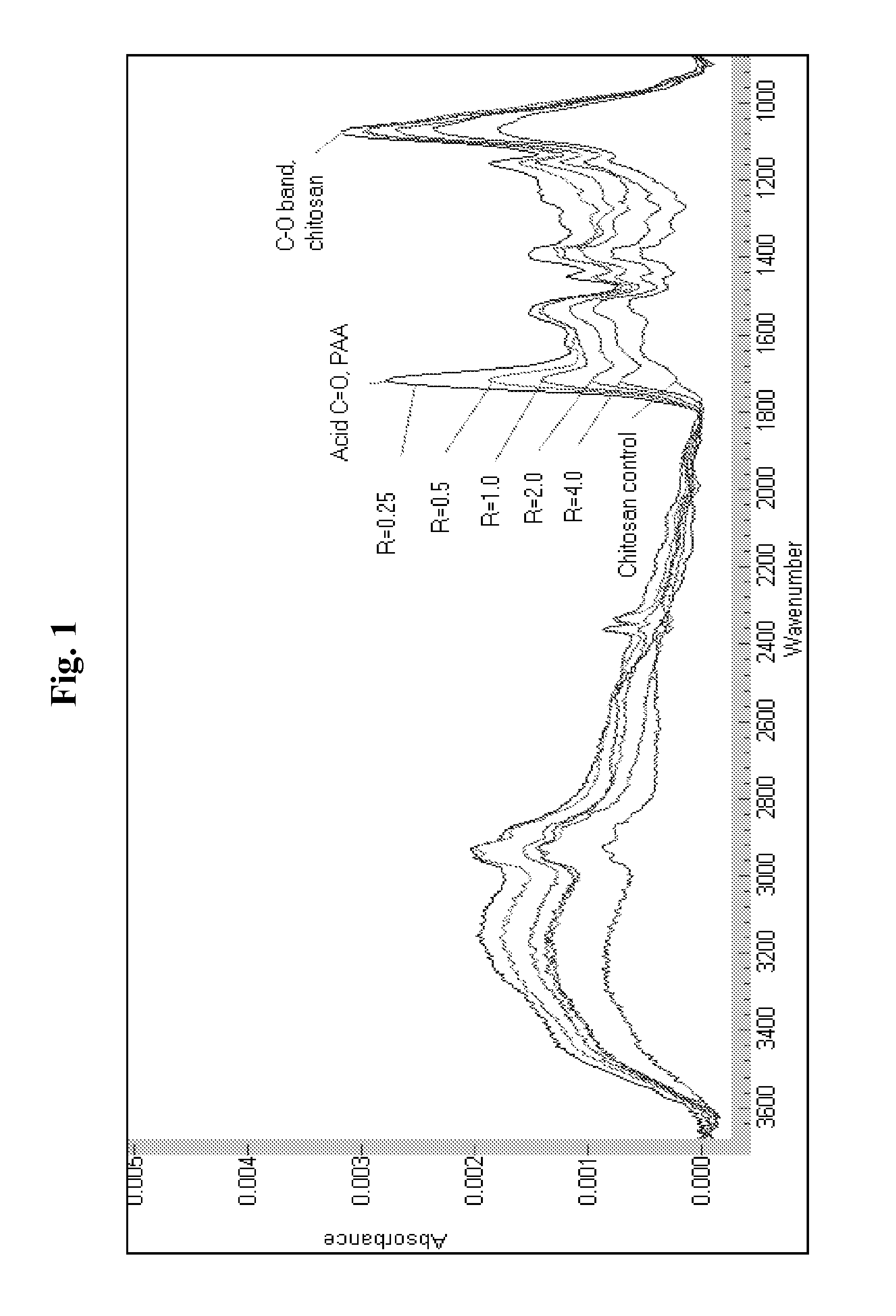
[0067]The size of the stable associative PECs, and the composition of the thin adsorbed layers formed by treating surfaces with aqueous solutions of PECs can be controlled by changing the ratio of the polymers comprising the associative PECs, i.e., by changing the R parameter.
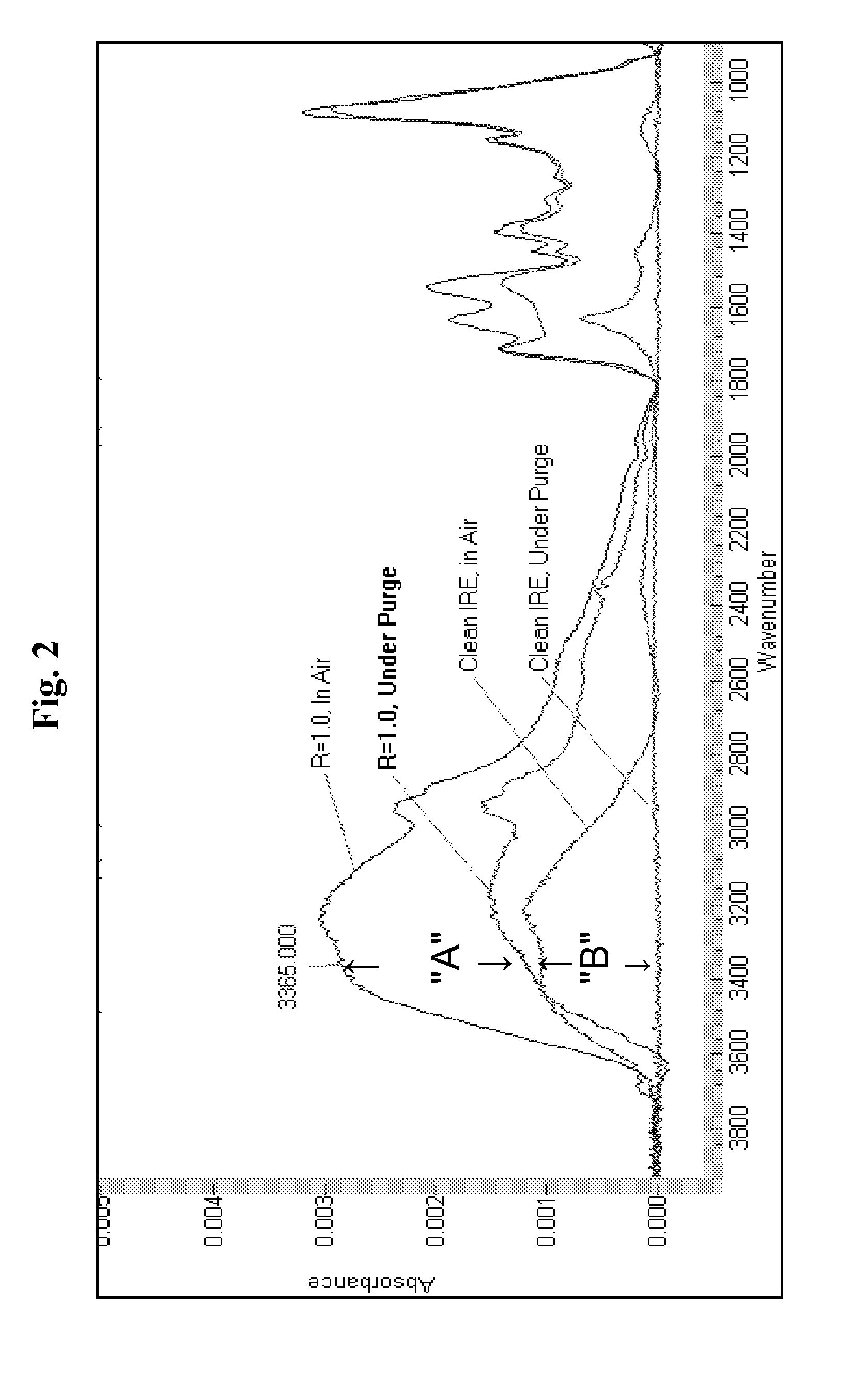
[0068]The FT-IR spectrum of chitosan, PAA and citric acid all exhibit one or more unique absorbance bands allowing their presence, as well as relative amount present on the surface of the IRE to be detected and monitored in real time.
[0069]The data in Table 2 illustrate that the composition of a layer can be controlled by varying the R parameter of the associative PECs. Since there were no added surfactants, the data also illustrate the surprising activity of the associative PECs solutions on a solid surface, even in the absence of a drying step and any “wetting” of the solid surface by surfactants.
[0070]Referring to Table 2, in one experiment with the R=0.25 associative PEC solution, the adsorption time was 5 ...
example 3
[0073]The associative PECs of the present invention, when made with polymers that exhibit chemical stability to oxidants such as sodium hypochlorite or hydrogen peroxide, are useful for providing hydrophilic modification of surfaces through the use of cleaning products familiar to consumers.
[0074]Hypochlorite-stable associative PECs can be made from mixtures of the alkali metal salt of poly(acrylic acid) (PAA) and poly(diallyl dimethyl ammonium chloride) denoted as poly(DADMAC) or simply DADMAC. However, the surface of a Ge IRE suitable for the FT-IR experiments is changed by exposure to sodium hypochlorite, which is a relatively strong oxidant. Thus, the formulations cited in Tables 3.1 and 3.2 below, which are used to demonstrate associative PEC stability and substantivity, were formulated using sodium chloride as a substitute for the sodium hypochlorite. It is believed, without being bound by theory, that the difference between the chloride and hypochlorite salts is immaterial, b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com