Oral Dosage Forms Of Bendamustine
a bendamustine and oral technology, applied in the field of oral dosage forms of bendamustine, can solve the problems of poor bioavailability, burdensome and time-consuming for healthcare professionals, and difficult reconstitution, and achieve the effect of improving the dissolution profile and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 1
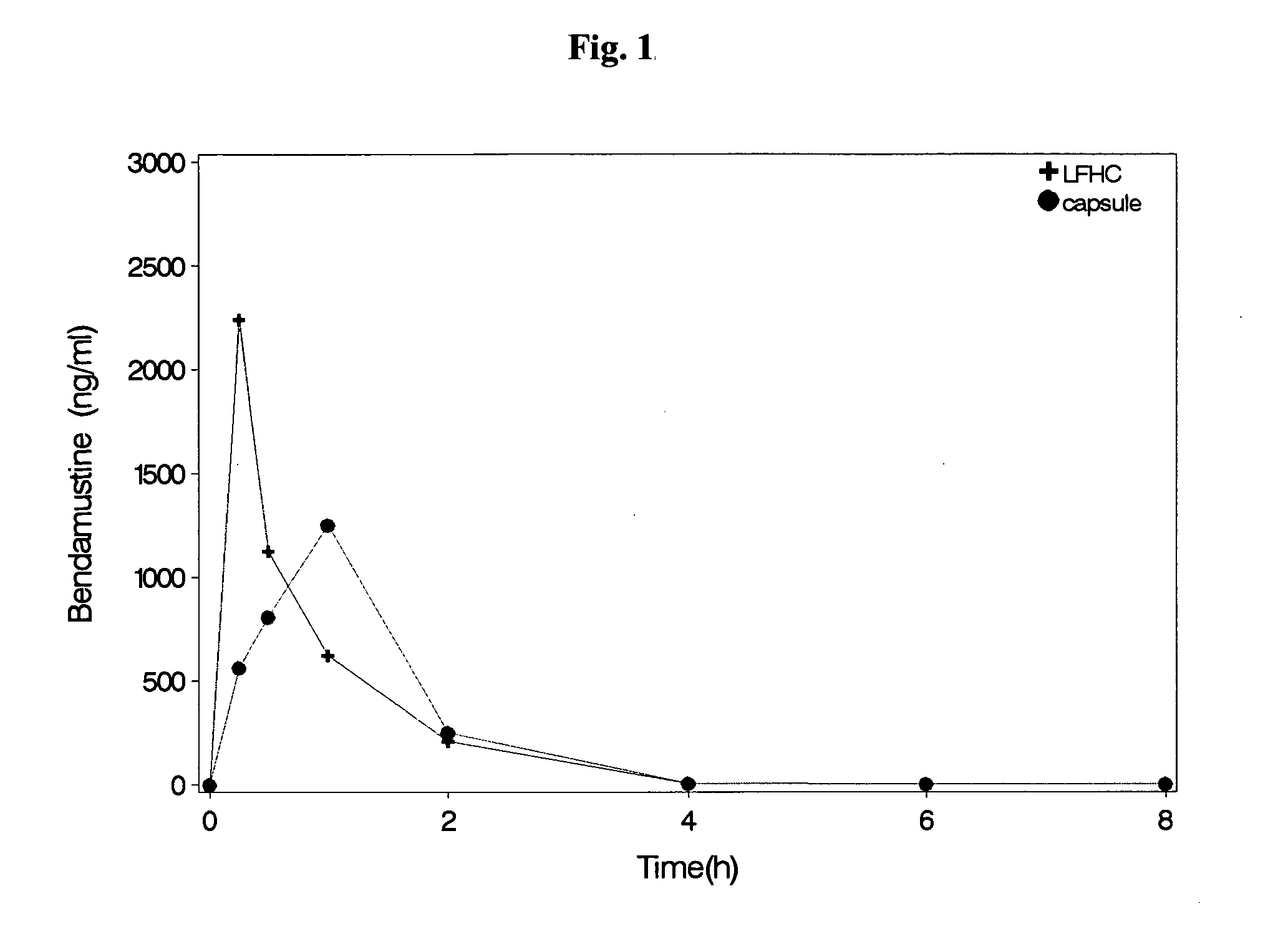
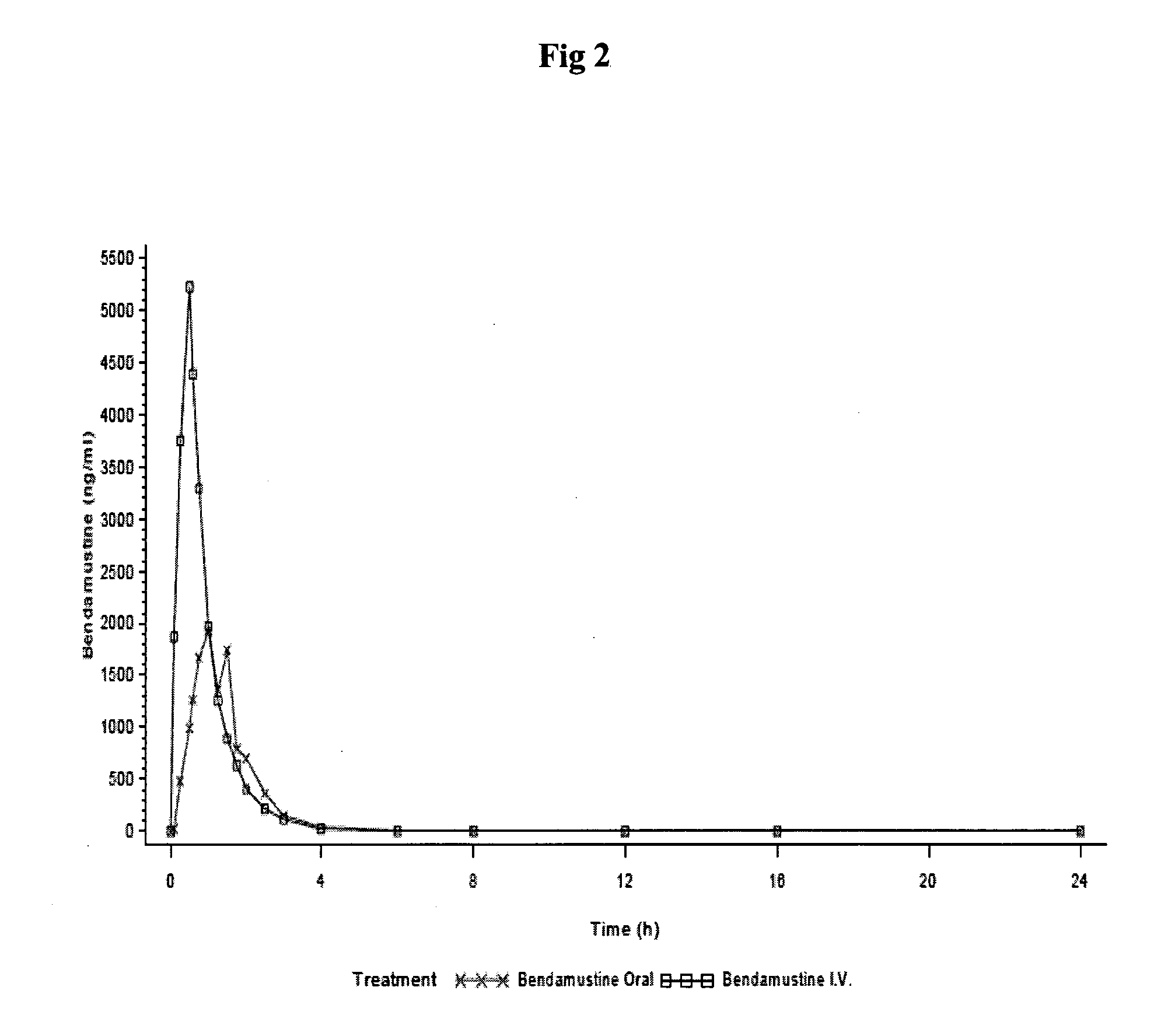
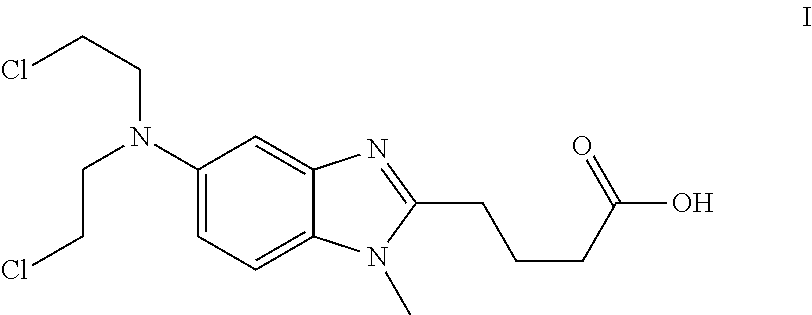
Bendamustine Capsule Formulation (Prior Art)
[0059]20.0±1 mg of bendamustine hydrochloride were weighed into the body of an empty hard gelatine capsule, and put into a clear glass HPLC vial (6 ml) of Agilent. Capsules were closed by placing the cap on top of the body and slight pushing.
[0060]Capsules were stored at 40° C. / 75% RH (glass vial open) or 50° C. (glass vial closed). The amount of bendamustine hydrochloride and of related substances was measured with HPLC (column: Zorbax Bonus-RP, 5 μm; temperature of column oven: 30° C.; temperature of autosampler: 5° C.; detector: 254 nm). The results are shown in Table 1:
TABLE 1Related substances and assay of bendamustine HCl(residual content) in bendamustine capsulesBendamustine HCl[% area]StorageRelatedT = 1T = 1conditionsubstancesT = 0monthT = 0month40° C. / HP10.100.4599.6498.8375% RHNP1*10.020.02(open vial)BM1Dimer*10.060.42BM1EE*10.130.11HP2 n.d.*2n.d.HP3n.d.n.d.50° C.HP10.101.4699.6497.51(closed vial)NP10.020.02BM1Dimer0.060.24BM1EE...
reference example 2
[0061]
TABLE 2aBendamustine powder mixture for capsulesComponentmg / dosage-formRelative Content %bendamustine hydrochloride55.121.09Mannitol141.454.11Microcrystalline cellulose25.09.57(Avicel ® PH101)Crosscarmellose sodium12.54.78(Ac-Di-Sol ®)Colloidal silicon dioxide1.00.38(Aerosil ® 200)Talc18.87.19Stearic acid7.52.87Sum261.3100
[0062]For a batch size of 1000 capsules all excipients except for colloidal silicon dioxide and stearic acid were loaded into a Somakon vessel (5 L). Bendamustine was added and blending was conducted for 4 minutes at 1000 rpm (wiper 10 rpm). The resulting blend was sieved through a 0.5 mm sieve. The vessel was reloaded with the blend and colloidal silicon dioxide was added. Blending was conducted for 2 minutes at the afore-mentioned conditions. Thereafter stearic acid was added and blending was continued for 1 minute. The blend was subsequently sieved through a 0.5 mm sieve, reloaded into the vessel and blended for another 30 seconds, all at the same conditio...
reference example 3
[0064]
TABLE 3aBendamustine powder mixture for capsulesComponentmg / dosage-formRelative Content %bendamustine hydrochloride55.121.09Lactose anhydrous141.454.11Microcrystalline cellulose25.09.57(Avicel ® PH112)Crosscarmellose sodium12.54.78(Ac-Di-Sol ®)Colloidal silicon dioxide1.00.38(Aerosil ® 200)Talc18.87.19Stearic acid7.52.87Sum261.3100
[0065]For 1000 capsules all excipients except for colloidal silicon dioxide and stearic acid were loaded into a Somakon vessel (5 L). Bendamustine was added and blending was conducted for 4 minutes at 1000 rpm (wiper 10 rpm). The resulting blend was sieved through a 0.5 mm sieve. The vessel was reloaded with the blend and colloidal silicon dioxide was added. Blending was conducted for 2 minutes at the afore-mentioned conditions. Thereafter stearic acid was added and blending was continued for 1 minute. The blend was subsequently sieved through a 0.5 mm sieve, reloaded into the vessel and blended for another 30 seconds, all at the same conditions.
[006...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pour point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com