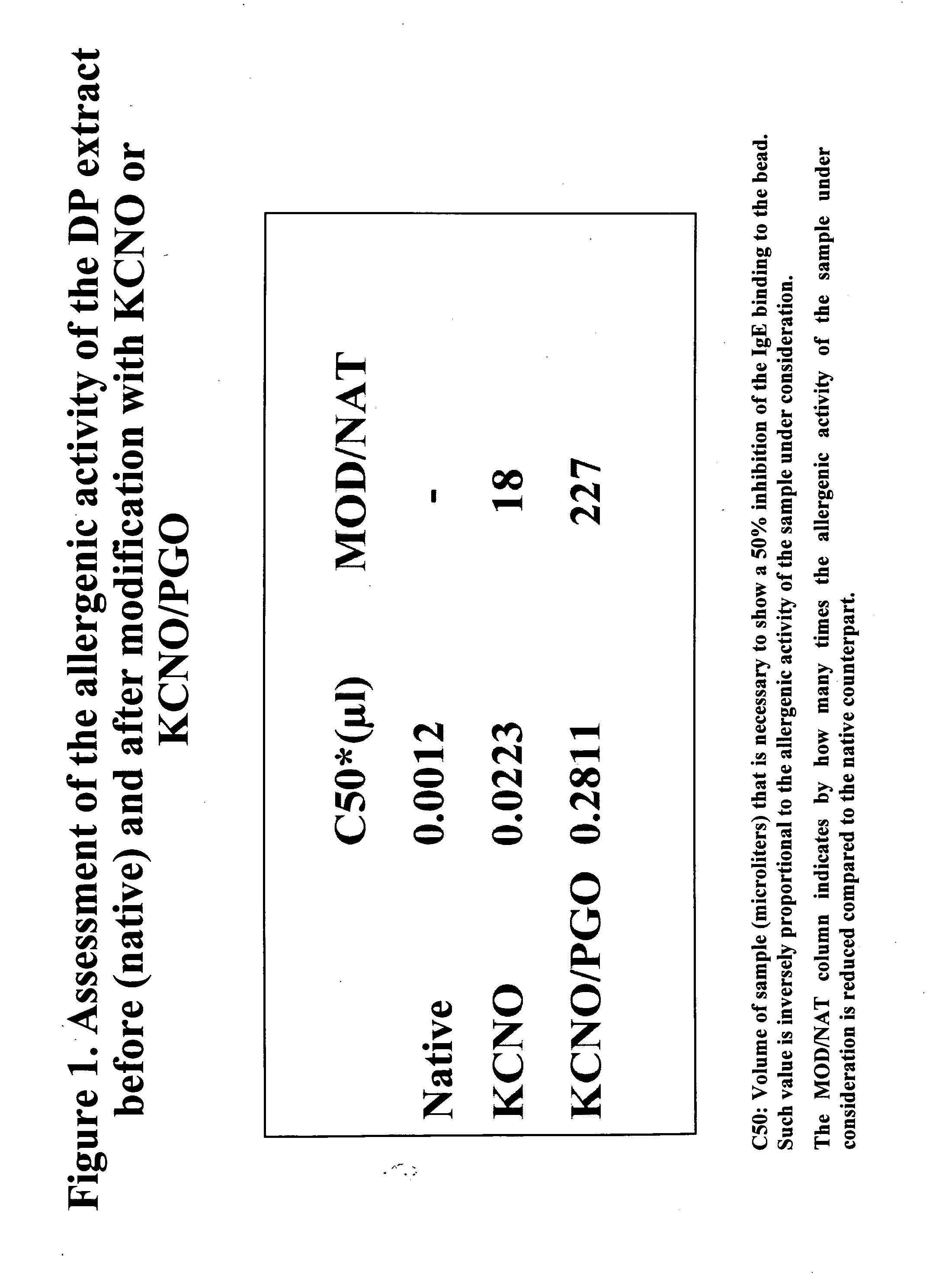
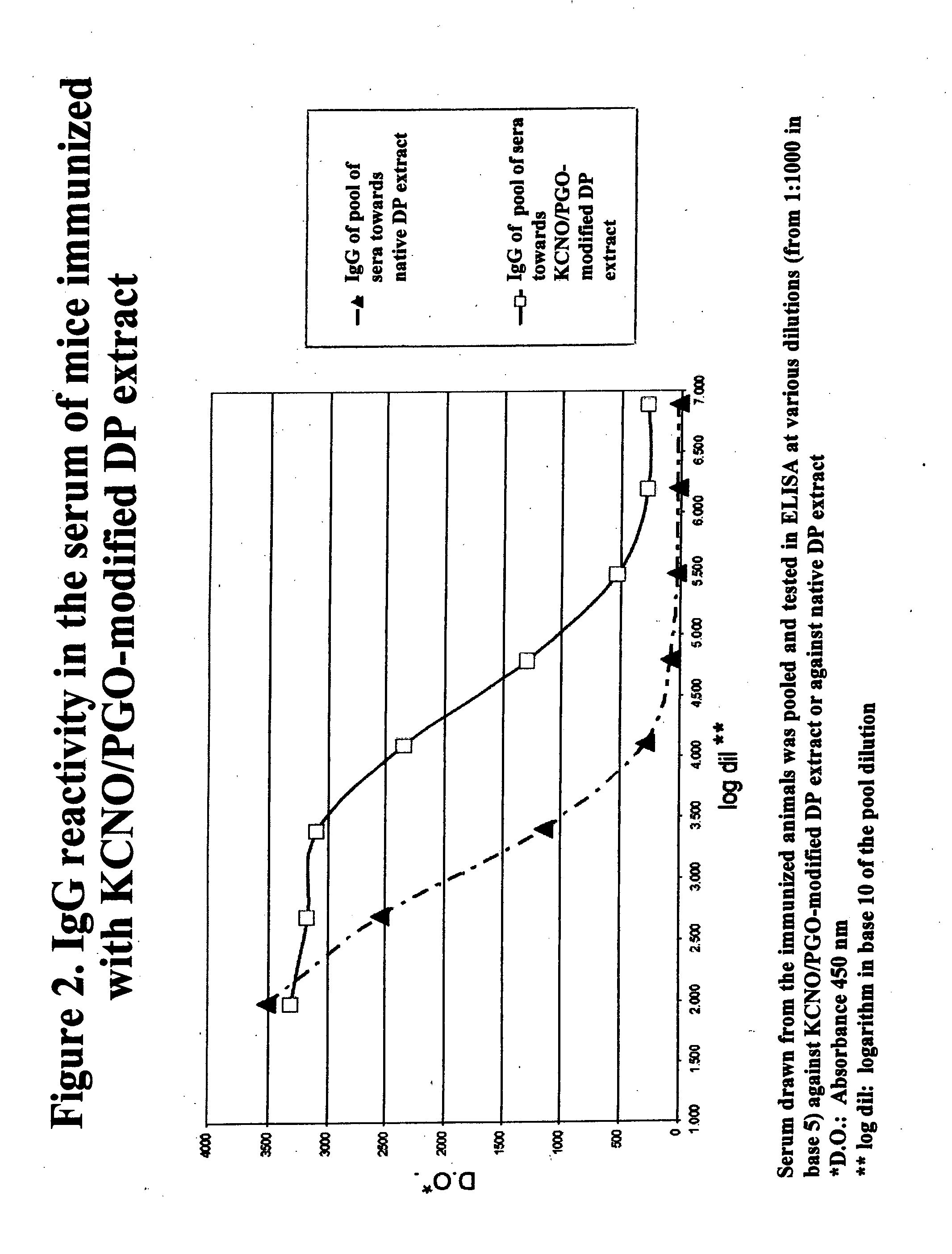
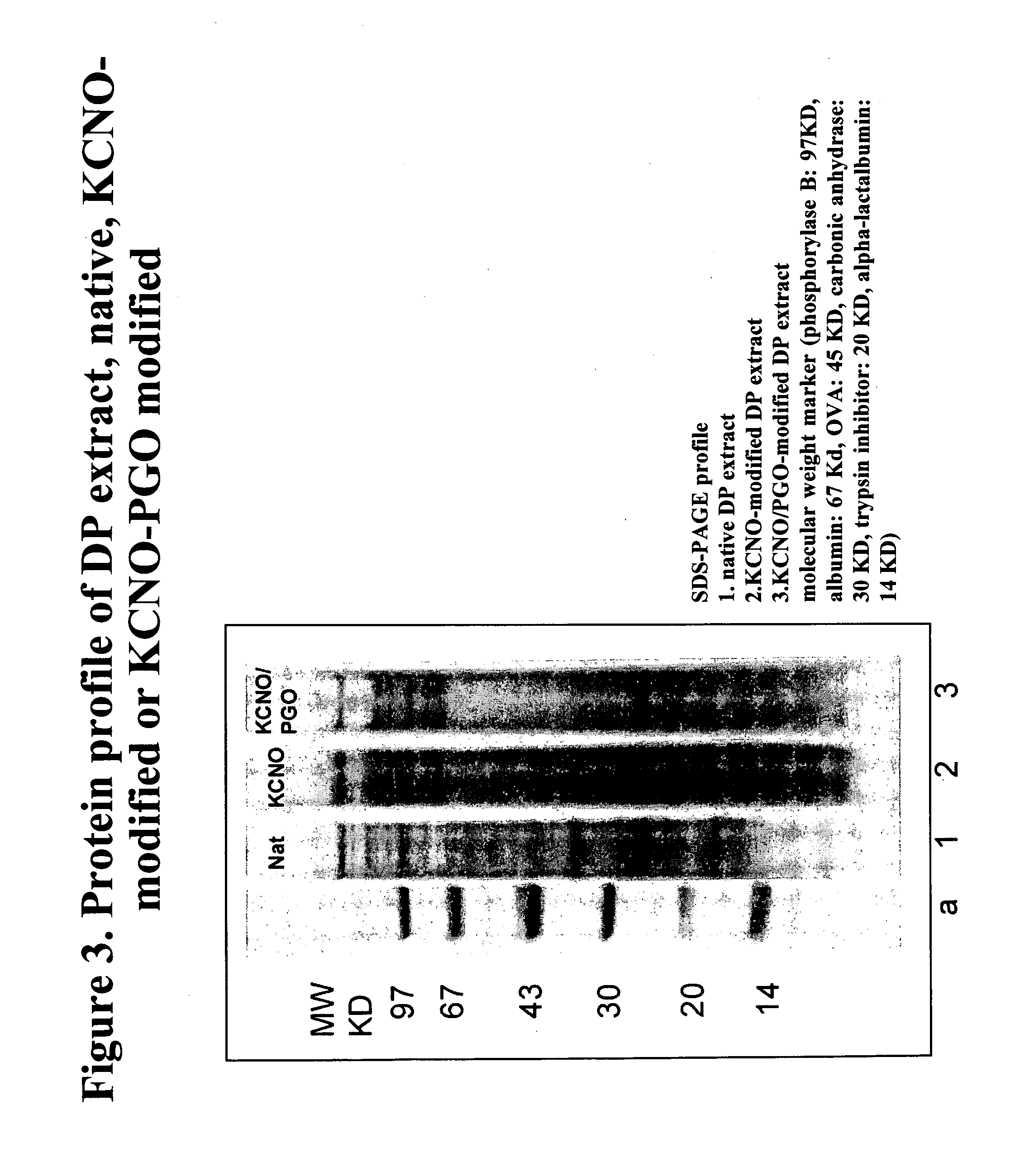
Allergoids Derived from Allergens
a technology of allergoids and allergens, applied in the field of preparation of allergoids derived from allergens, can solve the problems of not giving encouraging results on the reduction of allergenic potential, poor reduction of allergenic activity, etc., and achieves the effects of reducing the desensitization effect, reducing the risk of possible undesired effects, and reducing the risk of undesired effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Chemical Modification Procedure of an Extract of Mites of the Genus Dermatophagoides pteronissinus by KCNO, i.e., a KCNO / PGO Combination in Sequence
[0054]The extract of mites of the genus Dermatophagoides pteronissinus (Greer Labs, Lenoir, N.C., USA) has been prepared, after defatting with ethyl ether, by combining 100 mL of PBS (0.015 M phosphate buffer, 0.135 M NaCl, at pH 7.2) containing 0.05% azide (PBS-A) to 5 g of dehydrated mites bodies, and then subjecting the mixture to an ultrasonic treatment for 1 minute (Branson Ultrasonics, Sonifier 450, Darbury Conn., USA) in order to break the mites exoskeleton and to promote the extraction of the allergenic proteins contained therein. At the end, the preparation was put under stirring at 4° C. overnight. After centrifugation at 14000 rpm for 30 min and removal of the insoluble pellet, the surnatant was dialyzed against distilled H2O 2 and freeze dried.
[0055]The freeze dried extract is then taken up in a volume of 20 mM sodium phospha...
example 2
Chemical Modification Procedure of the Major Allergen Der p1 purified with KCNO, i.e., by a KCNO / PGO combination
[0069]a) Purification Step of the Der p1 Allergen from the DP Extract
[0070]The Der p1 allergen was purified from the DP extract by affinity chromatography, using a specific monoclonal antibody (isotype IgG1, produced at the Lofarma laboratories) covalently linked to a suitable matrix, such as CNBr-Sepharose (GE Helthcare, Milan), according to the procedure suggested by the manufacturer.
[0071]The Der p1 allergen, hold in a column, is eluted therefrom by using a buffer of 5 mM glycine, 50% ethylene glycol, pH 10.0. The purified allergen was quantified by spectrophotometric reading at 280 nm, by considering the molar extinction coefficient thereof (E280) as equal to 47330, hence an absorbance value, at a concentration of 1 mg / mL, equal to 1.89. Finally, the Der p1 was freeze dried in the presence of 1% saccharose.
[0072]The freeze dried Der p1 sample is then taken up in a volu...
example 3
Chemical Modification Procedure of the Major Allergen Ovalbumin (OVA) with KCNO, i.e., a KCNO / PGO Combination
[0083]A suitable amount of commercial OVA allergen (Sigma Aldrich, Milan), purified from egg albumen, is weighted and dissolved in a volume of 20 mM sodium phosphate buffer, pH 6.86, so as to reach a protein concentration of 2 mg / mL according to Lowry. For the modification with KCNO, 50.25 mg sodium tetraborate decahydrate and 101 mg potassium cyanate are added to 2.5 mL of OVA solution. The salts were brought to solution by slow stirring and the pH optionally adjusted to 9.3 with 1 M NaOH. The resulting solution was kept under slow stirring for 16 hours in a bath thermostated at 40° C. in a sealed flask. During the first hours, the pH was monitored and optionally adjusted by the addition of 1 M phosphoric acid. The thus-obtained preparation was gel-filtered again on G-25 column to remove the excess reagent, and sterilized on Millipore 0.22 micron membranes. A minimal part th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com