Methods of treating diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Clinical Trial and Analysis of Data
Methods and Methods
Patients, Treatment and Monitoring
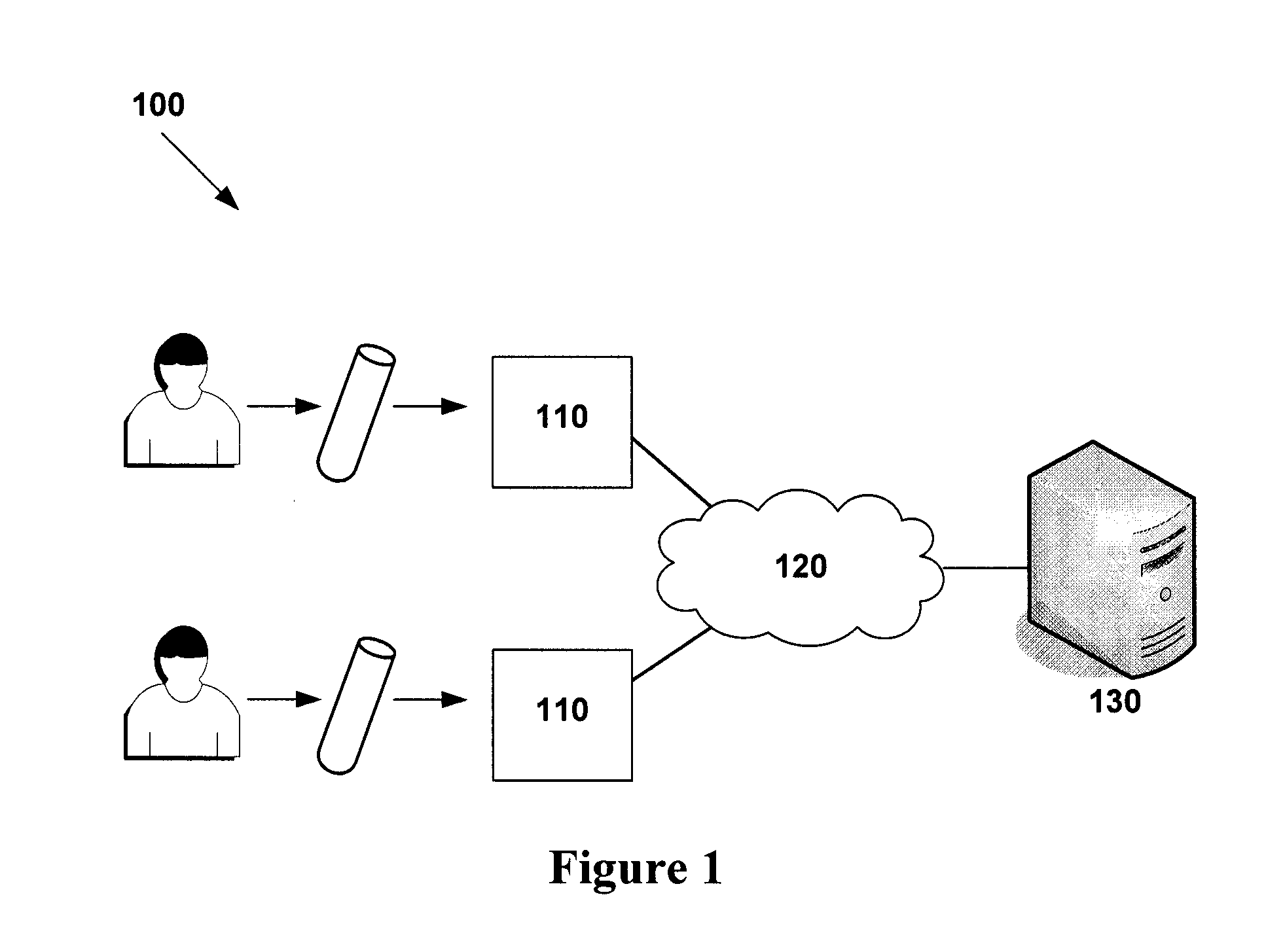
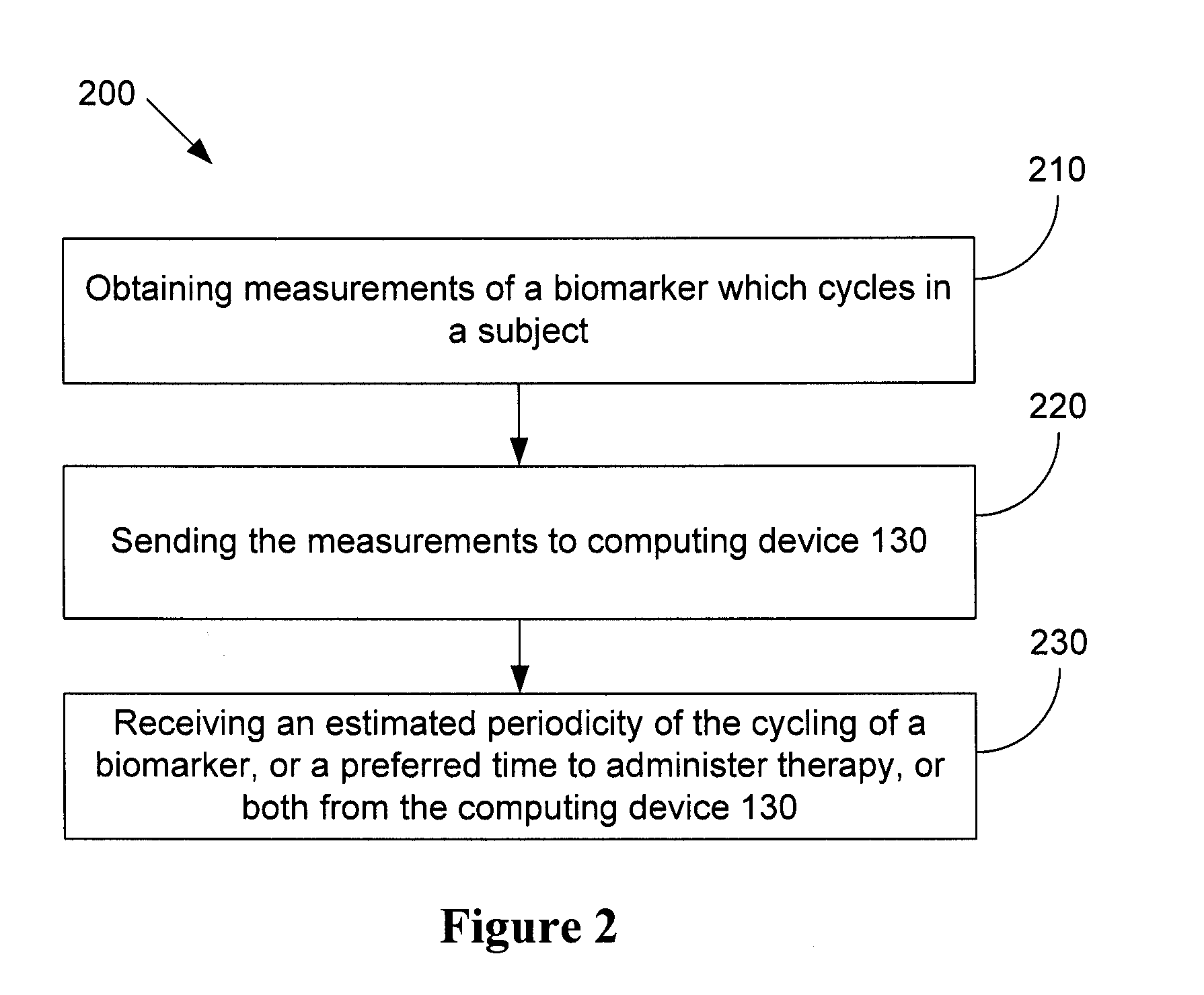
[0242]A pilot clinical study was conducted on 12 patients with metastatic melanoma (median age 61; 4 female; 7 with M1c disease) at The Mayo Clinic, Rochester, Minn., USA headed by Dr Svetomir Markovic. Serial CRP measurements were taken every 2-3 days for 2 weeks. The CRP oscillation cycle was identified by analysis of the raw data without any computer aided modelling, and chemotherapy with temozolomide (200 mg / m2 for 5 days, every 28 days) was initiated. Patients were evaluated for clinical and immune response endpoints every 8 weeks until progression.
Analysis of Immune System Cycling
[0243]In the described embodiment, the model form is:
log(CRPi)=cos(2π×(dayi-offsetperiod))×amplitude+mean+εi
That is, the natural logarithm CRP of a patient on day i is considered a harmonic function where the parameters (period, offset, mean, and amplitude) of the curve are unknown, and are estimated from the data....
example 2
Modelling to Predict Preferred Timing of Administration—Protocol Assessment
Introduction
[0284]The test of the software comprised two main portions: the use of the software on data from real and simulated patients and a simulation study. The overarching goal of the algorithm is to make the prediction as accurate as possible. The inventors can assess its ability to do so in simple, easy-to-grasp cases, as a means of developing intuition about how it will perform in complex cases that are harder to understand.
Simulated Patients
[0285]This example provides a demonstration of the use of the fitting algorithm on simulated patients.
[0286]Random patient were generated as follows:[0287]>p.random
[0288]It will be appreciated that random patients can be generated using any suitable statistical computing environment, such as open-source programming language R and MATLAB.
[0289]The random patient is then processed and reported using the following code ->report(p.random). Note that each simulated pa...
example 3
Simulation Study
Materials and Methods
[0304]The present inventors used the model and fitting algorithm as laid out in Example 1. The goal was to assess the impact upon prediction performance of the number of measures taken, the timeframe over which they were taken, and the pattern of spacing. It is reasonable to expect that the underlying variability of the patients biological signal would also affect the quality of the model fit. Therefore the design for the simulation study comprised the following elements:
[0305]1. Variation in length, including one, one and a half, and two weeks;
[0306]2. Variation in number of measurements, including 8, 10, 15, and 21;
[0307]3. Variation in measurement pattern, including symmetric (S), concentration early and late (B), and concentration late (L); and
[0308]4. Variation in underlying patient variability, including very small (0.25%) and nominal CRP variation (4%) to large (30%).
[0309]The inventors simulated 500 random patients with each of the three ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com