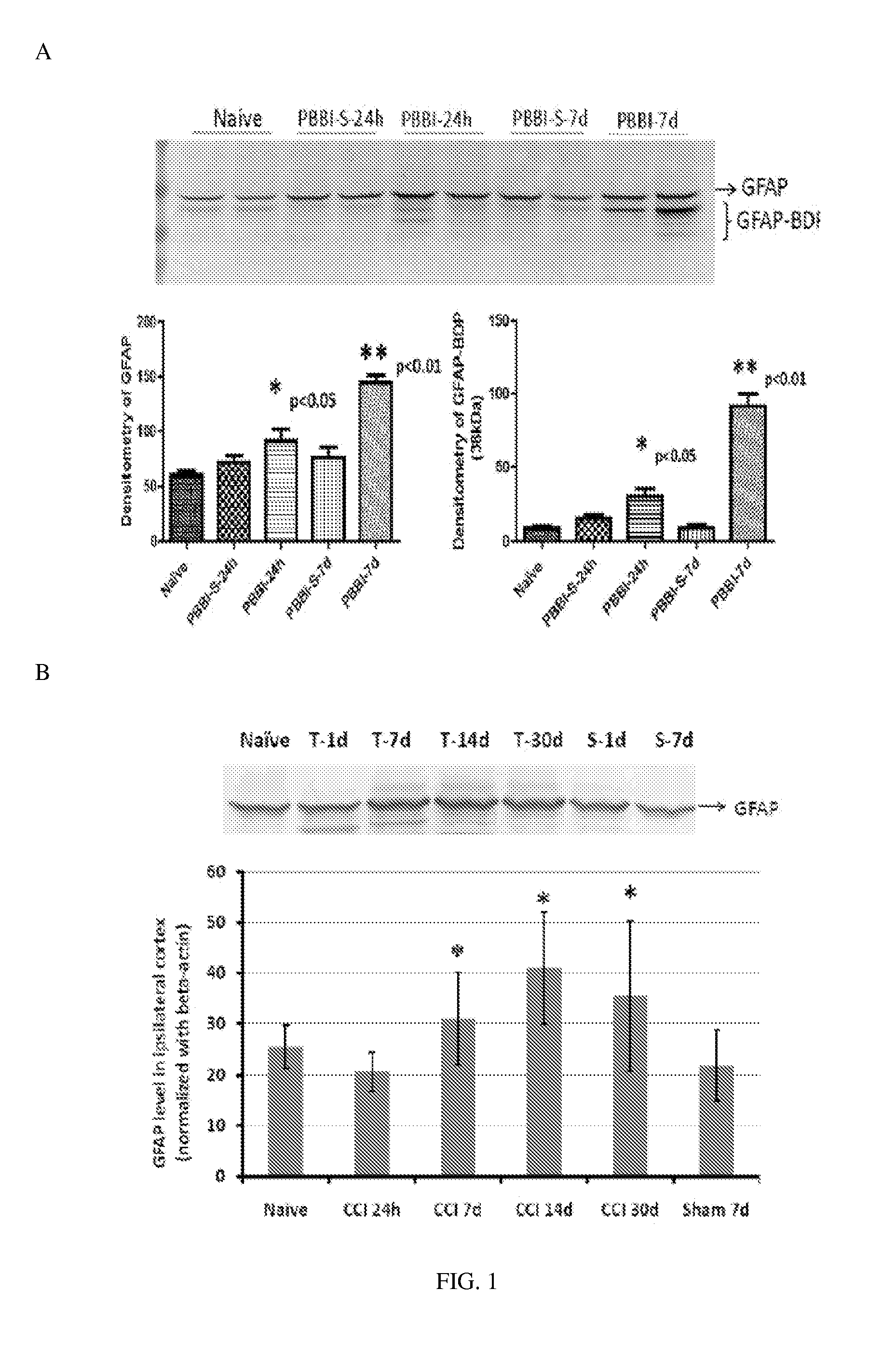
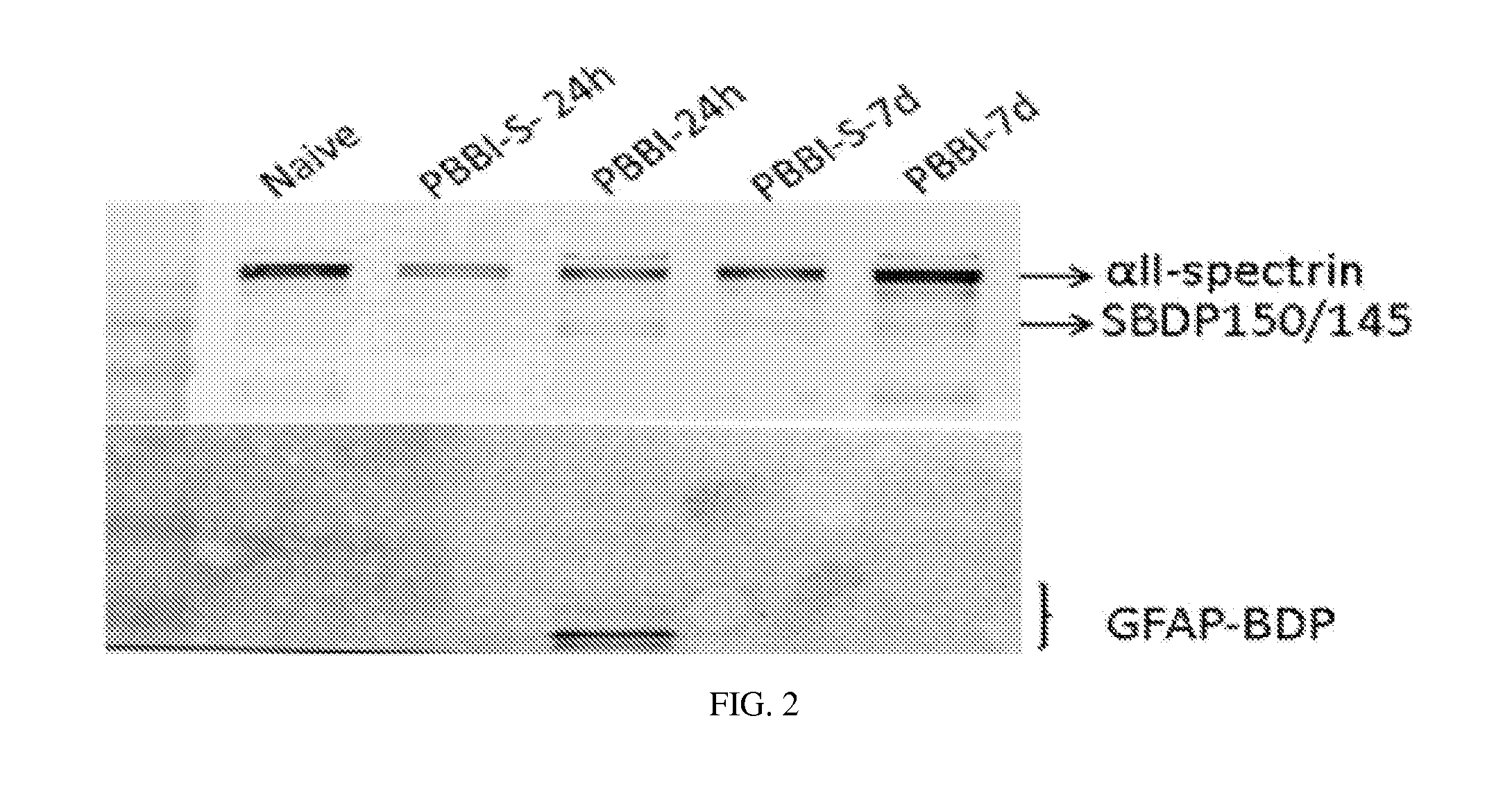
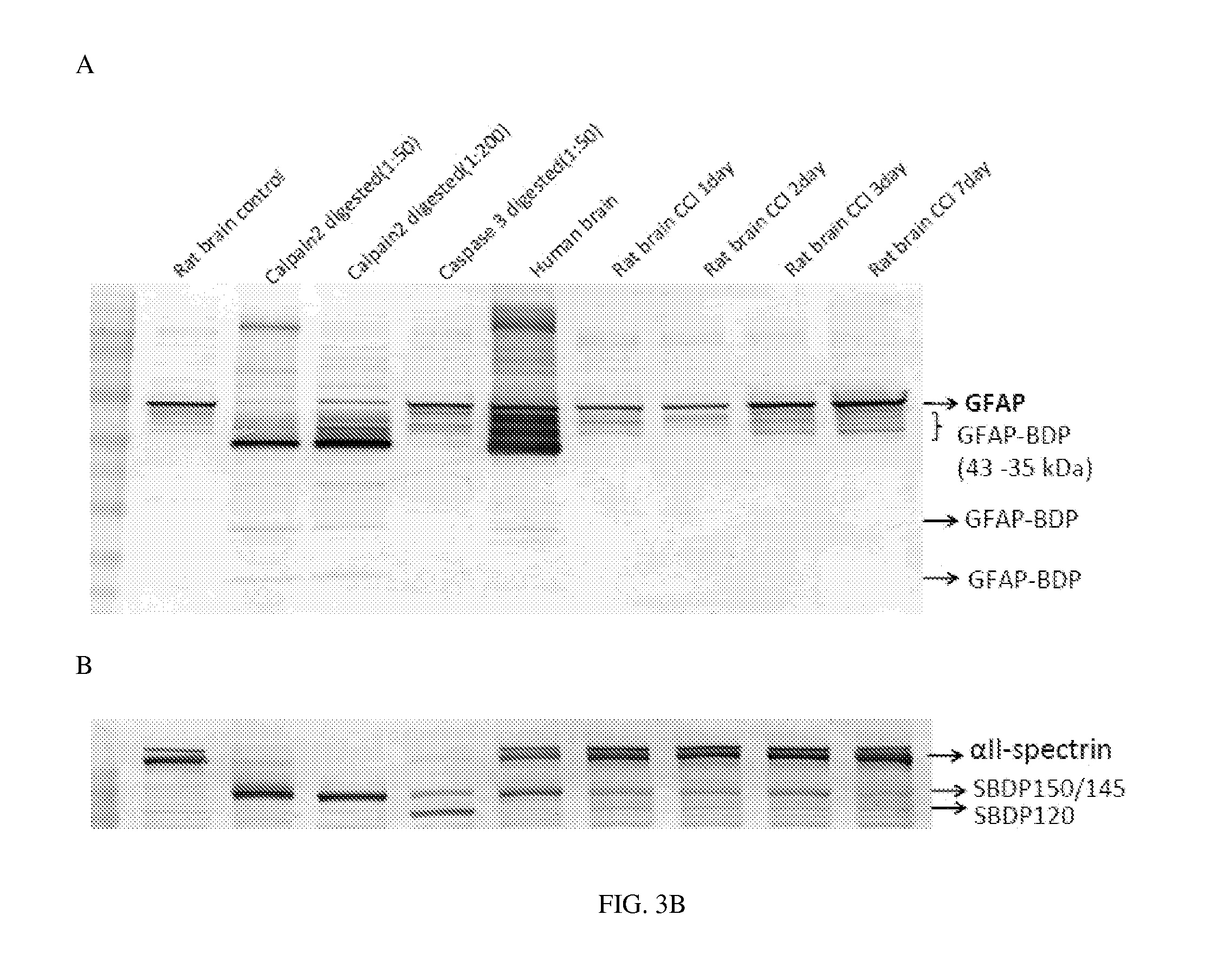
Micro-RNA, autoantibody and protein markers for diagnosis of neuronal injury
a neuronal injury and autoantibody technology, applied in the field of micro-rna, autoantibody and protein markers for diagnosis of neuronal injury, can solve the problems of limited value of clinical response testing of incapacitated individuals, high cost of spectroscopic imaging, and prognosis of brain damag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials for Biomarker Analyses
[0139]Illustrative reagents used in performing the subject invention include Sodium bicarbonate (Sigma Cat #: C-3041), blocking buffer (Startingblock T20-TBS) (Pierce Cat#: 37543), Tris buffered saline with Tween 20 (TBST; Sigma Cat #: T-9039). Phosphate buffered saline (PBS; Sigma Cat #: P-3813); Tween 20 (Sigma Cat #: P5927); Ultra TMB ELISA (Pierce Cat #: 34028); and Nunc maxisorp ELISA plates (Fisher). Monoclonal and polyclonal GFAP and UCHL1 antibodies are made in-house or are obtained from Santa Cruz Biotechnology, Santa Cruz, Calif. Antibodies directed to α-II spectrin, GFAP, and breakdown products as well as to MAP2, MBP, neurofascin, IgG, and IgM are available from Santa Cruz Biotechnology, Santa Cruz, Calif.
[0140]The anti-tau antibody directed to full length tau is purchased from Santa Cruz Biotechnology, Santa Cruz, Calif. To generate antibodies specific to tau-BDPs, the synthetic peptide (Cys-C6-SIDMVD—COOH) (SEQ ID NO: 1) which is the seq...
example 2
Biomarker Assay Development
[0142]Anti-biomarker specific rabbit polyclonal antibody and monoclonal antibodies as well as antigens are produced in the laboratory or purchased commercially. To determine reactivity specificity of the antibodies to detect a target biomarker a known quantity of isolated or partially isolated biomarker is analyzed or a tissue panel is probed by western blot. An indirect ELISA is used with the recombinant biomarker protein attached to the ELISA plate to determine optimal concentration of the antibodies used in the assay. Microplate wells are coated with rabbit polyclonal anti-human biomarker antibody. After determining the concentration of rabbit anti-human biomarker antibody for a maximum signal, the lower detection limit of the indirect ELISA for each antibody is determined. An appropriate diluted sample is incubated with a rabbit polyclonal antihuman biomarker antibody for 2 hours and then washed. Biotin labeled monoclonal anti-human biomarker antibody ...
example 4
Middle Cerebral Artery Occlusion (MCAO) Injury Model
[0144]Rats are incubated under isoflurane anesthesia (5% isoflurane via induction chamber followed by 2% isoflurane via nose cone), the right common carotid artery (CCA) of the rat is exposed at the external and internal carotid artery (ECA and ICA) bifurcation level with a midline neck incision. The ICA is followed rostrally to the pterygopalatine branch and the ECA is ligated and cut at its lingual and maxillary branches. A 3-0 nylon suture is then introduced into the ICA via an incision on the ECA stump (the suture's path was visually monitored through the vessel wall) and advanced through the carotid canal approximately 20 mm from the carotid bifurcation until it becomes lodged in the narrowing of the anterior cerebral artery blocking the origin of the middle cerebral artery. The skin incision is then closed and the endovascular suture left in place for 30 minutes or 2 hours. Afterwards the rat is briefly reanesthetized and the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com