Signal amplification system based on bioluminescence resonance energy transfer and detection method thereof
A resonance energy transfer and bioluminescence technology, applied in the field of biomedical analysis, can solve the problems of low detection sensitivity and inability to achieve detection signal amplification, and achieve the effect of improving detection sensitivity and good versatility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Preparation of Energy Donor Protein and Energy Acceptor Protein
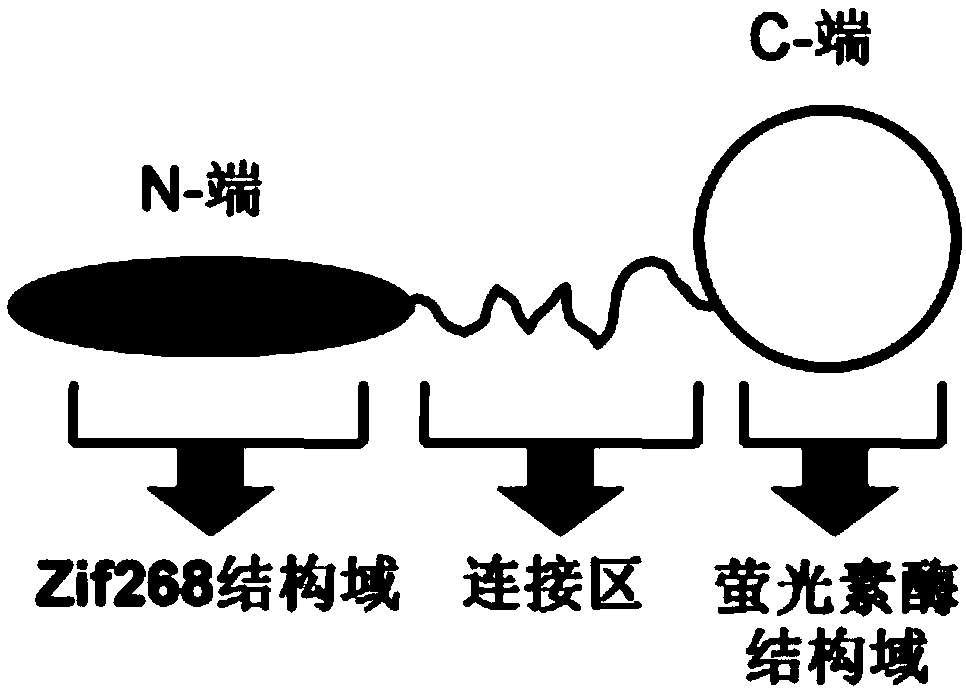
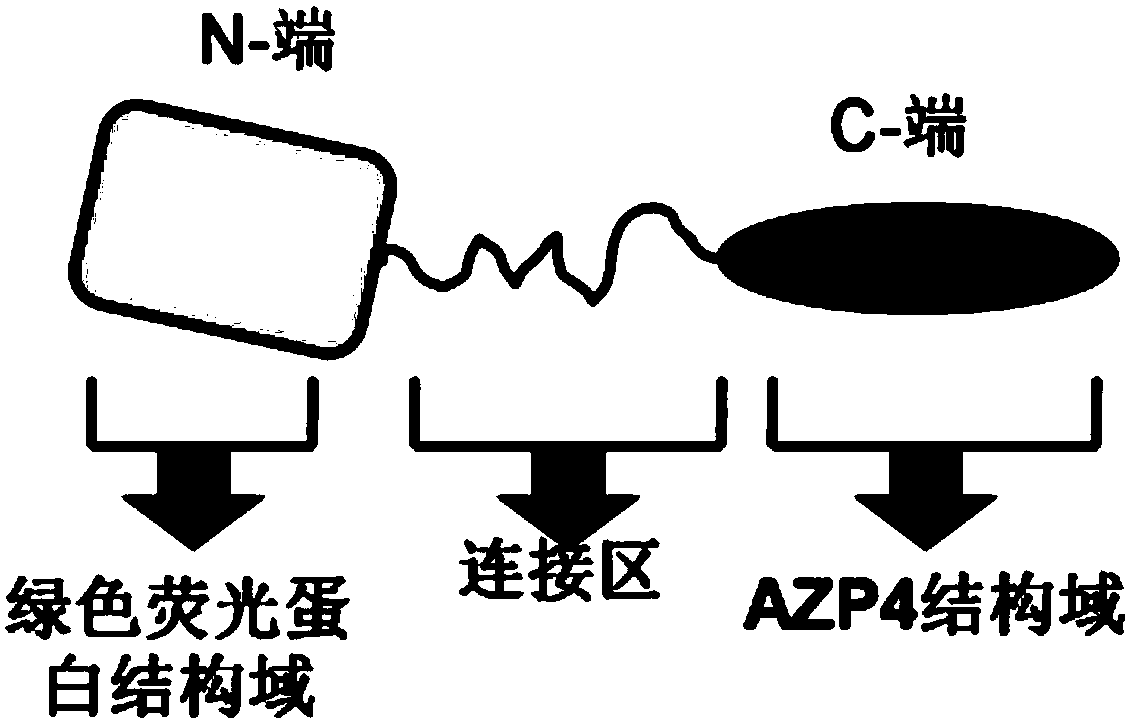
[0039] The structure of the energy donor protein in the present invention is as figure 1 As shown, the structure of the energy acceptor protein is as figure 2 Shown, the preparation method of both is as follows:
[0040] (1) Amplification of the gene encoding the energy donor protein: firstly, the artificially synthesized luciferase coding fragment was amplified by PCR with primers P1 and P2, and then the artificially synthesized zinc finger domain Zif268 was encoded by primers P3 and P4 The fragments were amplified by PCR. The amplified fragments were recovered by agarose gel electrophoresis, mixed in equimolar amounts, and finally the full-length gene fragment encoding the energy donor protein was obtained by overlapping PCR amplification using P3 and P2 as primers, and the obtained full-length gene Fragments were purified by agarose gel electrophoresis and stored in a -20°C refrigerator ...
Embodiment 2
[0046] Example 2: Signal Amplification of Bioluminescence Resonance Energy Transfer
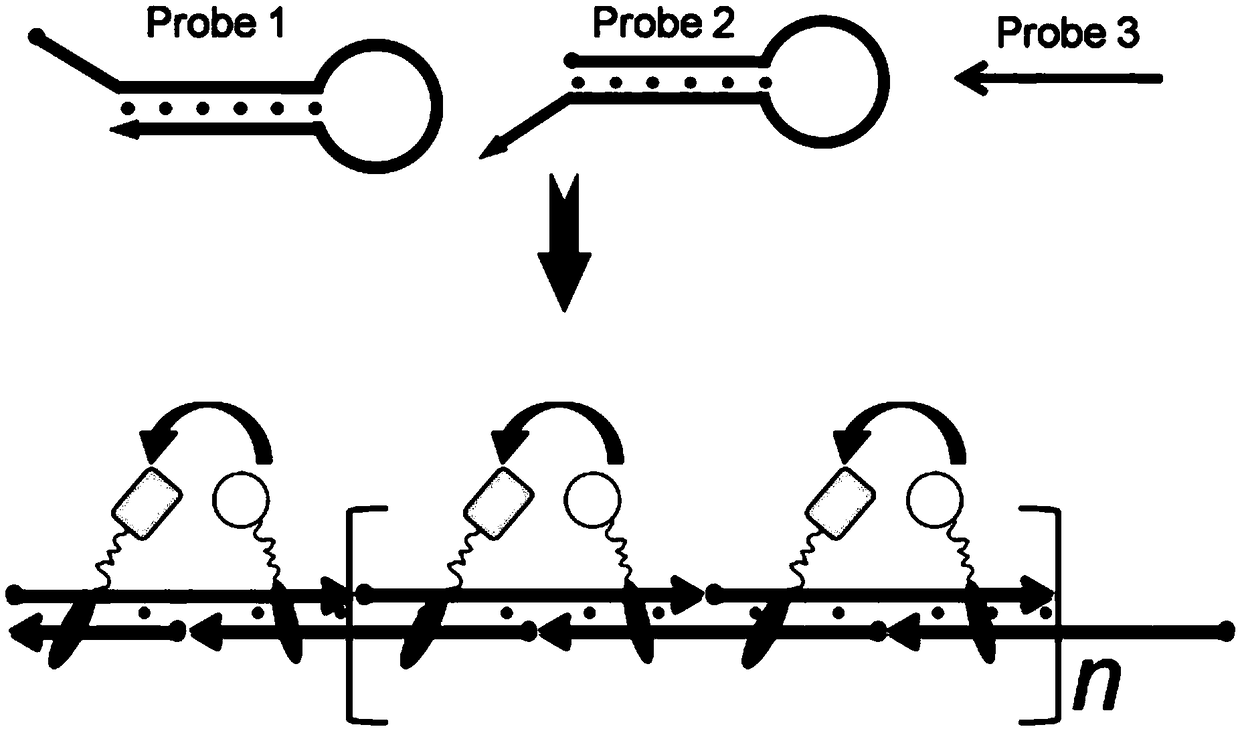
[0047] The principle of signal bioluminescence resonance energy transfer signal amplification provided by the present invention is as follows: image 3 As shown, the principle of its reaction system preparation and signal detection is as follows:
[0048] (1) Preparation of reaction system: Mix 50nM probe1, 50nM probe2, 50nM energy donor protein, 250nM energy acceptor protein, and 5nM probe3 in 100μL of ZnK buffer (20mM Tris-Cl, 100mM NaCl, 5mM MgCl 2 , 0.1 mM ZnCl 2 , 1 mM TCEP, 0.1 mg / mL bovine serum albumin (BSA), pH=7.4), and incubated at room temperature for 30 min.
[0049] (2) Signal detection: add an equal volume of luciferase detection buffer (purchased from Promega Company) to the above solution, detect by a spectrometer, turn off the light source of the spectrometer during detection, and the detection wavelength range is 400nm to 600nm, and the detection result can be obtained I...
Embodiment 3
[0050] Example 3: Detection of α-thrombin
[0051] (1) Preparation of nucleic acid aptamer immunomagnetic beads: Take 5 mg of amino-modified magnetic beads (particle size: 300 nm) and incubate with 50 mM Sulfo-SMCC for 1 h. (10mM HEPES, 5mM EDTA, pH=7.2) washed 3 times, took 1mg of Sulfo-SMCC activated magnetic beads and 10μM nucleic acid aptamer A1 and incubated in 200μL HEPES buffer for 4 hours, then washed with 5% (w / v) BSA Seal overnight and store in a refrigerator at 4°C until use.
[0052] (2) Detection of α-thrombin: Take 0.5 mg / mL aptamer immunomagnetic beads prepared in step (1), incubate with 0.1 μM aptamer A2 and the sample to be tested at room temperature for 30 min, After separating the magnetic beads with 200 μL ZnK buffer three times, incubate the magnetic beads with 50 nM probe1, 50 nM probe2, 50 nM energy donor protein, and 250 nM energy acceptor protein for 30 min, and add an equal volume of luciferase to the above solution The detection buffer is detected ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com