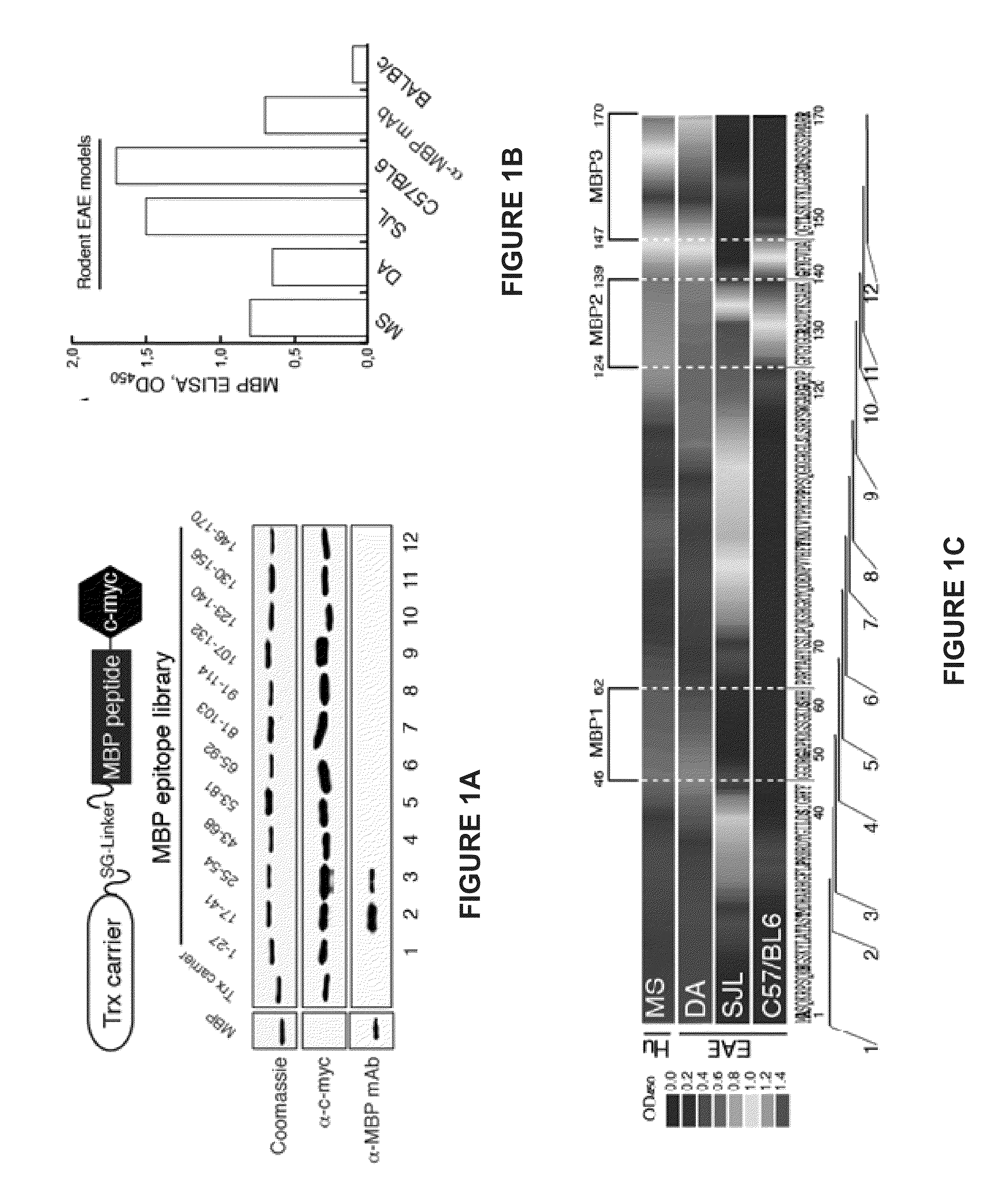
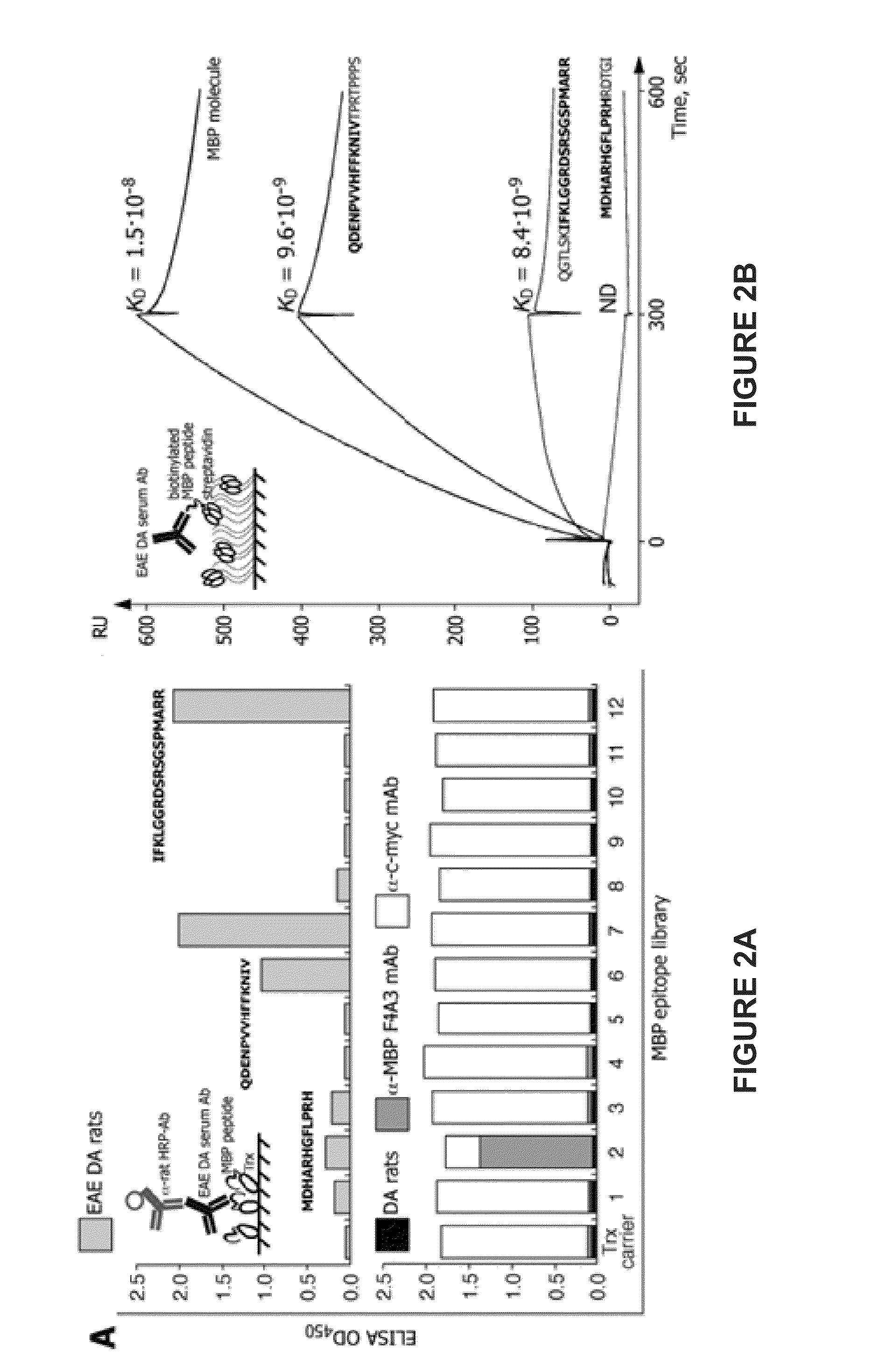
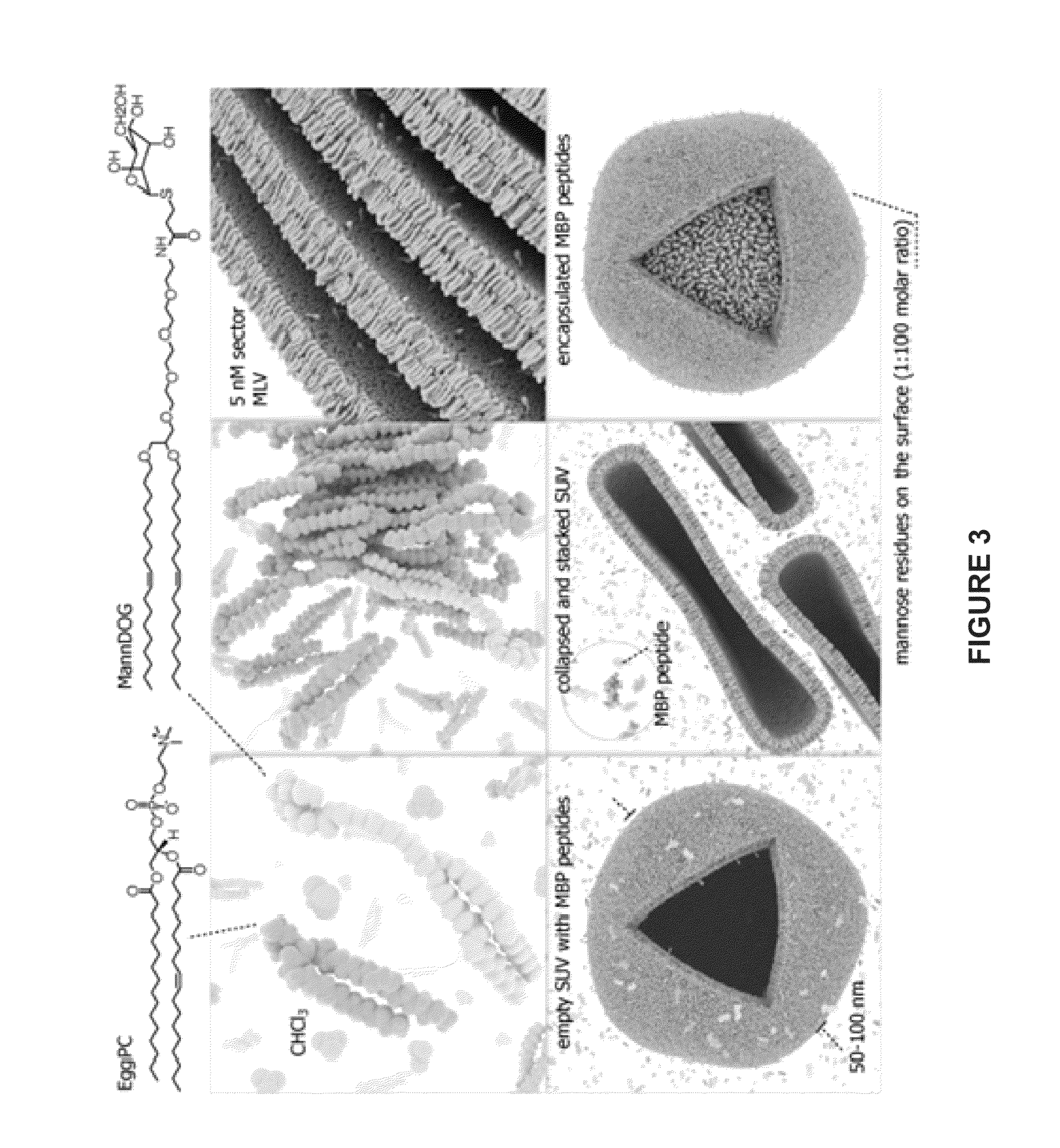
Liposomes containing oligopeptide fragments of myelin basic protein, a pharmaceutical composition and a method for treatment of multiple sclerosis
a technology of myelin basic protein and liposome, which is applied in the direction of peptide/protein ingredients, peptide sources, immunodeficiency disorders, etc., can solve the problems of inflammatory demyelinating lesions in the brain and spinal cord, no existing therapies are capable of curing or preventing ms progression, and the formation of demyelination scarring and other problems, to achieve the effect of reducing paralysis, improving the intake of thes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Liposomes Containing MBP Peptides by Sonication
[0226]45 g of a phospholipid mixture containing 1 part by weight of mannosylated DOG lipid (ManDOG) and 99 parts by weight of 2,3-dipalmitoyl-sn-glycero-1-phosphatydyl choline was dissolved in 450 mL of chloroform and placed in a 1 L vacuum evaporator flask. The chloroform was evaporated under vacuum to form a lipid film on the flask walls. After evaporation, the flask was filled with nitrogen gas, and 800 mL of water for injections (WFI) was slowly introduced therein. The flask was placed in the ultrasonic bath for 30 minutes to disrupt pre-formed liposomes. Liposomes re-formed after sonication, resulting in an aqueous emulsion of liposomes.
[0227]0.75 g of an MBP peptide mixture containing GGDRGAPKRGSGKDSHH (SEQ ID NO:1; MBP1), GFGYGGRASDYKSAHK (SEQ ID NO:2; MBP2), and QGTLSKIFKLGGRDSRSGSPMARR (SEQ ID NO:3; MBP3) and excess lactose (lactose to lipid ratio of 3:1) in equal amounts was then dissolved in 40 mL of water for ...
example 2
Preparation of Liposomes Containing MBP Peptides Via Disintegration
[0229]45 g of a phospholipid mixture containing 1 part by weight of mannosylated DOG lipid (ManDOG) and 99 parts by weight of 2,3-dipalmitoyl-sn-glycero-1-phosphatydyl choline was dissolved in 450 mL of chloroform and placed in a 1 L vacuum evaporator flask. The chloroform was evaporated under vacuum to form a lipid film on the flask walls. After evaporation, the flask was filled with nitrogen gas, and 800 mL of water for injections was slowly introduced therein. The resulting mixture is transferred to a flowing disintegrator receiver and the stroke volume pressure of the disintegrator is set at 150 MPa. 100 ml. of the mixture is added to the flowing disintegrator per load, and the resulting liposomal emulsion is collected from the disintegrator receiver.
[0230]0.75 g of an MBP peptide mixture containing GGDRGAPKRGSGKDSHH (SEQ ID NO:1; MBP1), GFGYGGRASDYKSAHK (SEQ ID NO:2; MBP2), and QGTLSKIFKLGGRDSRSGSPMARR (SEQ ID N...
example 3
Aqueous Formulation of Liposomes Containing MBP Peptides
[0232]100 mL of buffered phosphate saline solution (FBR) was added to 1000 mg of lyophilized MBP-peptide liposomes, prepared as described in Example 1, under sterile conditions and stirred. Beta-carotene was added then added to the composition at a final concentration of 0.01%, as an antioxidant. The liposome composition was then dispensed into hydrolytic class I glass containers under sterile conditions and a nitrogen atmosphere. The containers were subsequently sealed with rubber plugs and fitted with aluminum caps.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com