Efficient, self sufficient production of methanol from a methane source via oxidative bi-reforming
a technology of methanol and methane, which is applied in the direction of ether preparation, ether preparation, biofuels, etc., can solve the problems of significant amounts of carbon dioxide or other oxidation byproducts, and achieve the effects of significant cost savings, careful control conditions, and additional costly steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
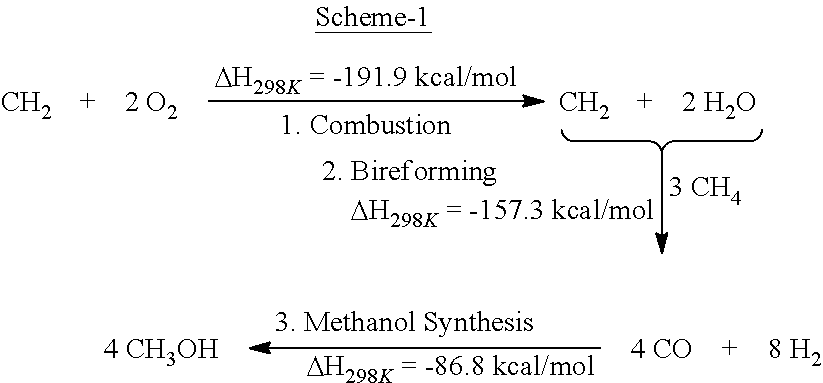
[0037]One equivalent of methane is subjected to complete oxidation, followed by the bi-reforming process of the effluents with added three equivalents of methane in a suitable double walled flow reactor over metal / metal oxide catalysts of such as NiO at a temperature of about 800° C. to 1100° C., preferentially between 800-850° C. Suitable catalysts also include varied metal and metal oxides such as V, Ti, Ga, Mg, Cu, Ni, Mo, Bi, Fe, Mn, Co, Nb, Zr, or Sn used as single metal, metal oxides or their combination. They can be supported on suitable support, preferentially suitably large nanostructured surface such as fumed silica or alumina and are thermally activated under hydrogen. A preferred catalyst is NiO on fused alumina support. This process provides metgas, the desired mixture of CO and H2.
example 2
[0038]Adjusting the feed mixture to natural(shale) gas or other methane sources in Example 1 to give CO and H2 composition of 2:1 mole ratio (metgas) suitable for the production of methanol. The feed, when needed is also purified from excess CO2, H2S and other impurites.
example 3
[0039]A metgas mixture of hydrogen and carbon monoxide produced in approximately 2:1 ratio is converted to produce methanol under catalytic reaction conditions using usual copper and zinc oxides or related catalysts.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com