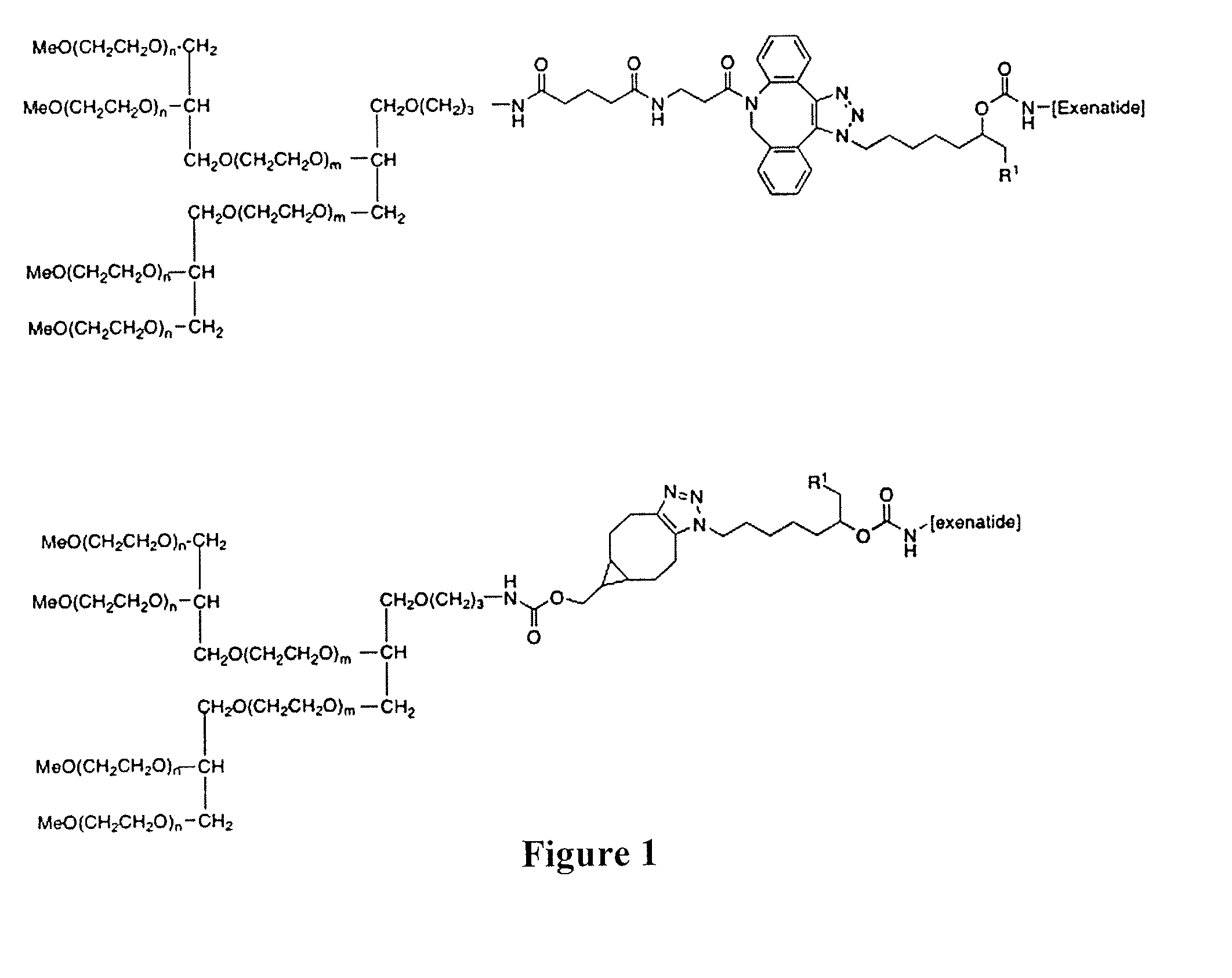
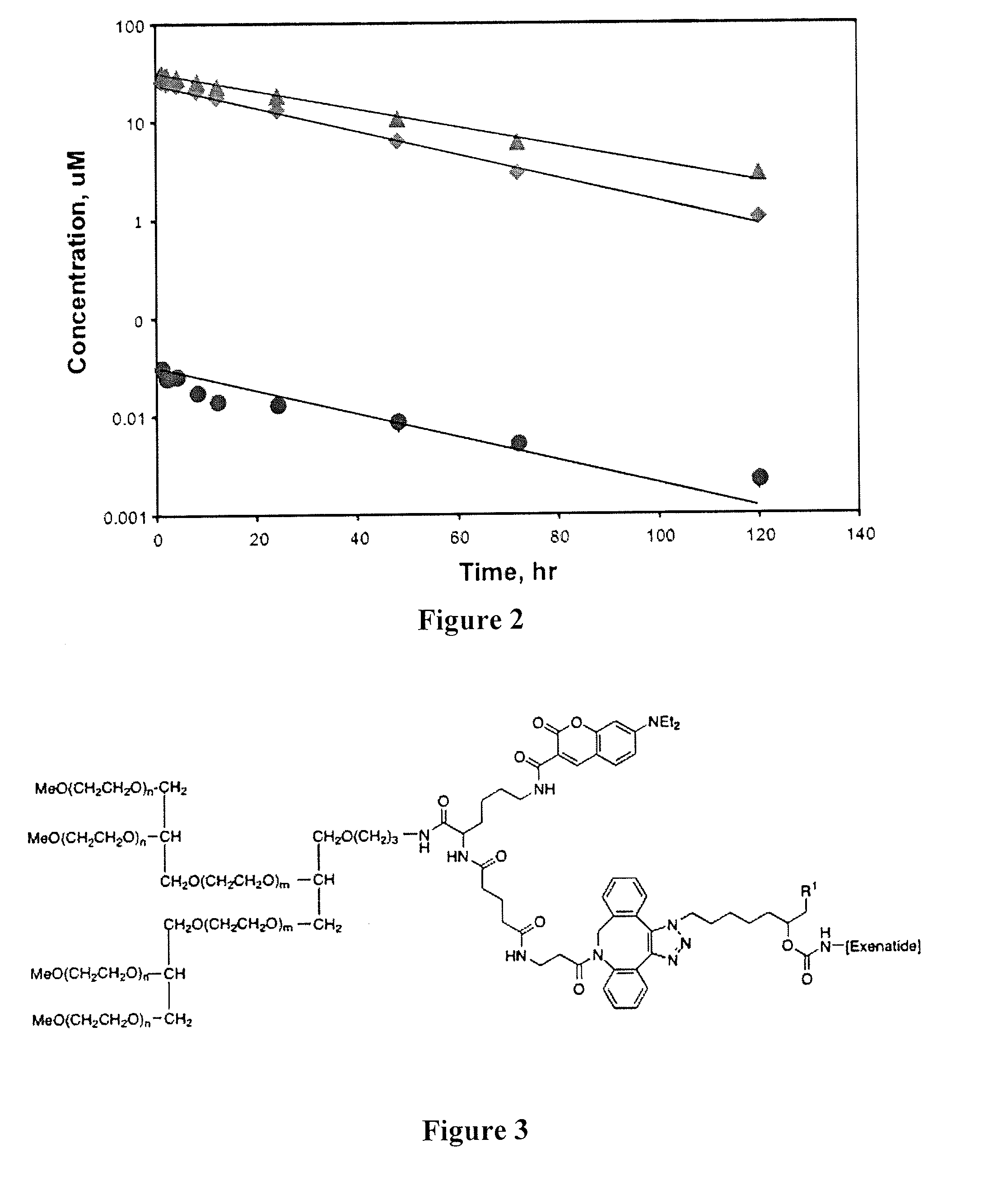
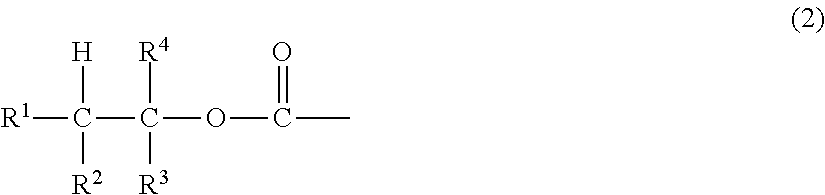Peg conjugates of exenatide
a technology of exenatide and conjugates, which is applied in the field of slow release conjugates of exenatide, can solve the problems of short circulating half-life and ineffectiveness of glp-1 as a therapeutic agen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Azido-Linker-OSu
[0065]
[0066](1) 6-azido-1-hexanol: a mixture of 6-chloro-1-hexanol (25 g, 183 mmol) and sodium azide (32.5 g, 500 mmol) in 200 mL of water was heated at reflux for 20 h, then cooled to ambient temperature and extracted 3× with ethyl acetate. The combined extracts were washed with brine, dried over MgSO4, filtered, and concentrated to yield the product as a pale yellow oil (28.3 g).
[0067](2) 6-azidohexanal: Solid trichloroisocyanuric acid (4.3 g) was added in small portions to a vigorously stirred mixture of 6-azido-1-hexanol (7.15 g), TEMPO (50 mg), and sodium bicarbonate (5.0 g) in dichloromethane (100 mL) and water (10 mL). The mixture was stirred for an additional 30 minutes after addition, then filtered through a pad of Celite. The organic phase was separated and washed successively with sat. aq. NaHCO3 and brine, then dried over MgSO4, filtered, and concentrated to provide the product (5.8 g), which was used without further purification.
[0068](3) ...
example 2
Preparation of Azido-Linker-Exenatides
[0081]
[0082]Fmoc-TentaGel® Rink amide resin (0.17 meq / g) was used to synthesize protected exenatide using standard Fmoc / tBu chemistry. The resin was allowed to swell in dichloromethane and then washed with N,N-dimethylformamide (DMF). Fmoc groups were removed using piperidine. Coupling steps were performed using O-benzotriazole-N,N,N′,N′-tetramethyl-uronium-hexafluoro-phosphate (HBTU) and N-methylmorpholine in DMF. After removal of the final Fmoc group and washing, the resin was treated with 3 molar equivalents (based on resin substitution) of the azido-linker-OSu (Example 1) and 3 molar equivalents of N,N-diisopropyl-ethylamine in DMF. Reaction progress was followed by ninhydrin test. After 2 h, the resin was washed with methanol and ether, then dried. Cleavage and deprotection of the product used a mixture of trifluoroacetic acid / triisopropylsilane / water for 4 h. The crude product was then precipitated with ether, and purified by HPLC using a ...
example 3
Preparation of GL4-400-DBCO
[0089]
[0090]A solution of a 4-branched mPEG-amine (GL4-400-PA, NOF Corporation) of mw=40 kDa (1.0 g, 25 μmol) in 10 mL of THF was reacted with DBCO-NHS (24 mg, 50 μmol) and triethylamine (7 μL) for 24 h at ambient temperature. Precipitation into 50 mL of MTBE provided 0.9 g of the 4-branched GL4-400-PA-DBCO.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com