Fusion protein comprising an interleukin 4 and interleukin
a technology of interleukin and fusion protein, which is applied in the field of immunology and pharmacology, can solve the problems of limited therapeutic intervention, modification of the contour of adjacent articulating surfaces, and not all patients respond well to such type of therapeutic intervention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Transfection of HEK293 Cells
[0124]Method:
[0125]HEK293 cells were transiently transfected according to standard procedures with a vector containing a transgene (Y Derocher et al., Nucleic Acids Research 2002, vol 30, no 2, e9). Briefly, the IL4-IL10 fusion protein insert was cloned in a pUPE expression vector, containing a cystatin signal sequence. HEK293E cells were then transfected with the pUPE expression vector containing the IL4-IL10 fusion protein of the present invention. At the same time cells were co-transfected with a vector carrying the transgene for beta-galactoside alpha-2,3-sialyltransferase 5 (SIAT 9) homo sapiens to optimize capping of the glycans with sialic acid. Cells were cultured in FreeStyle medium (Invitrogen) with 0.9% primatone and ˜0.04%, v / v, fetal calf serum. Five days after transfection, the conditioned medium was collected by low-speed centrifugation, after which it was concentrated over a 10 kDa QuixStand hollow fibre cartridge (GE Healthcare) and diafi...
example 2
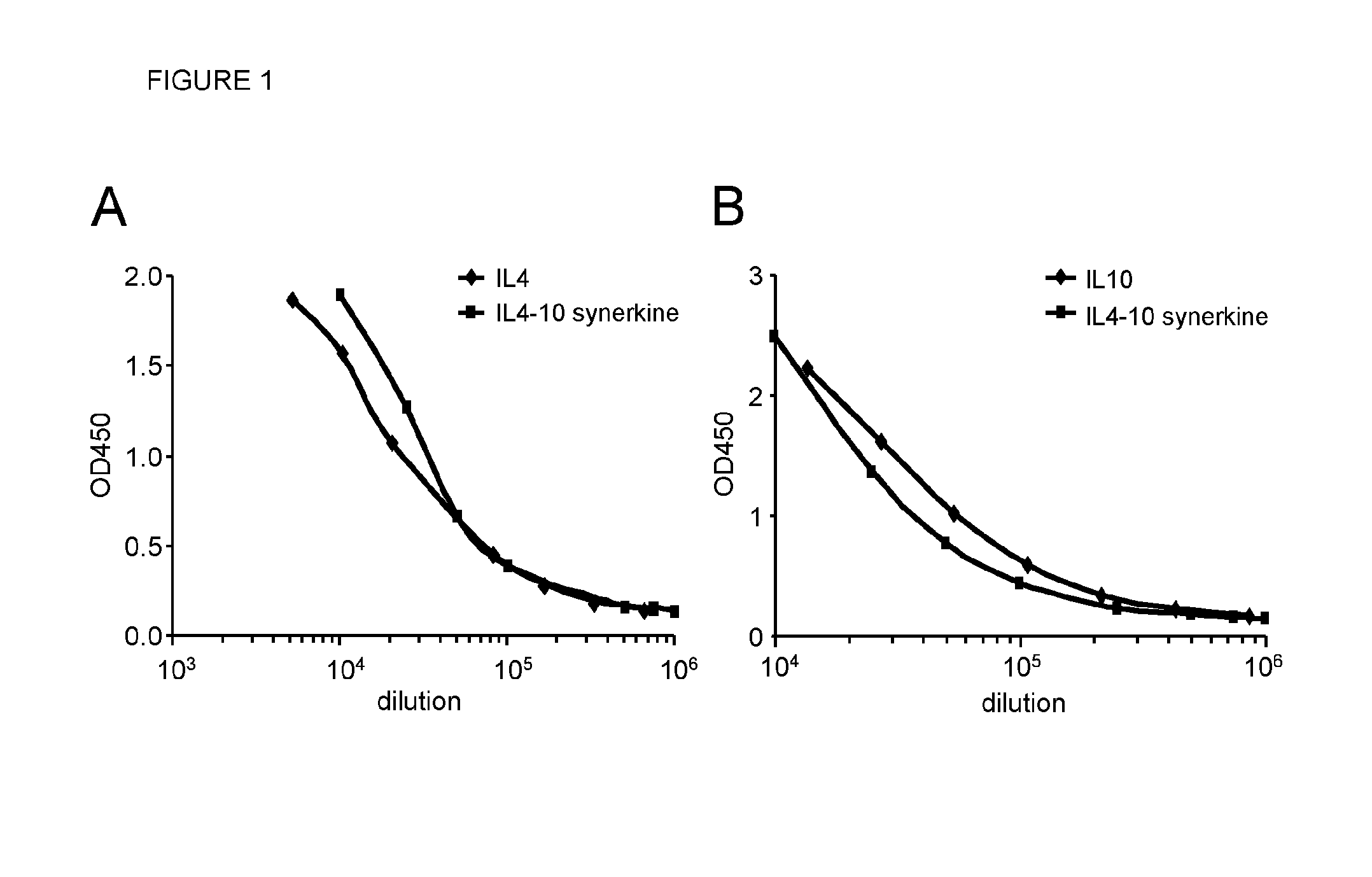
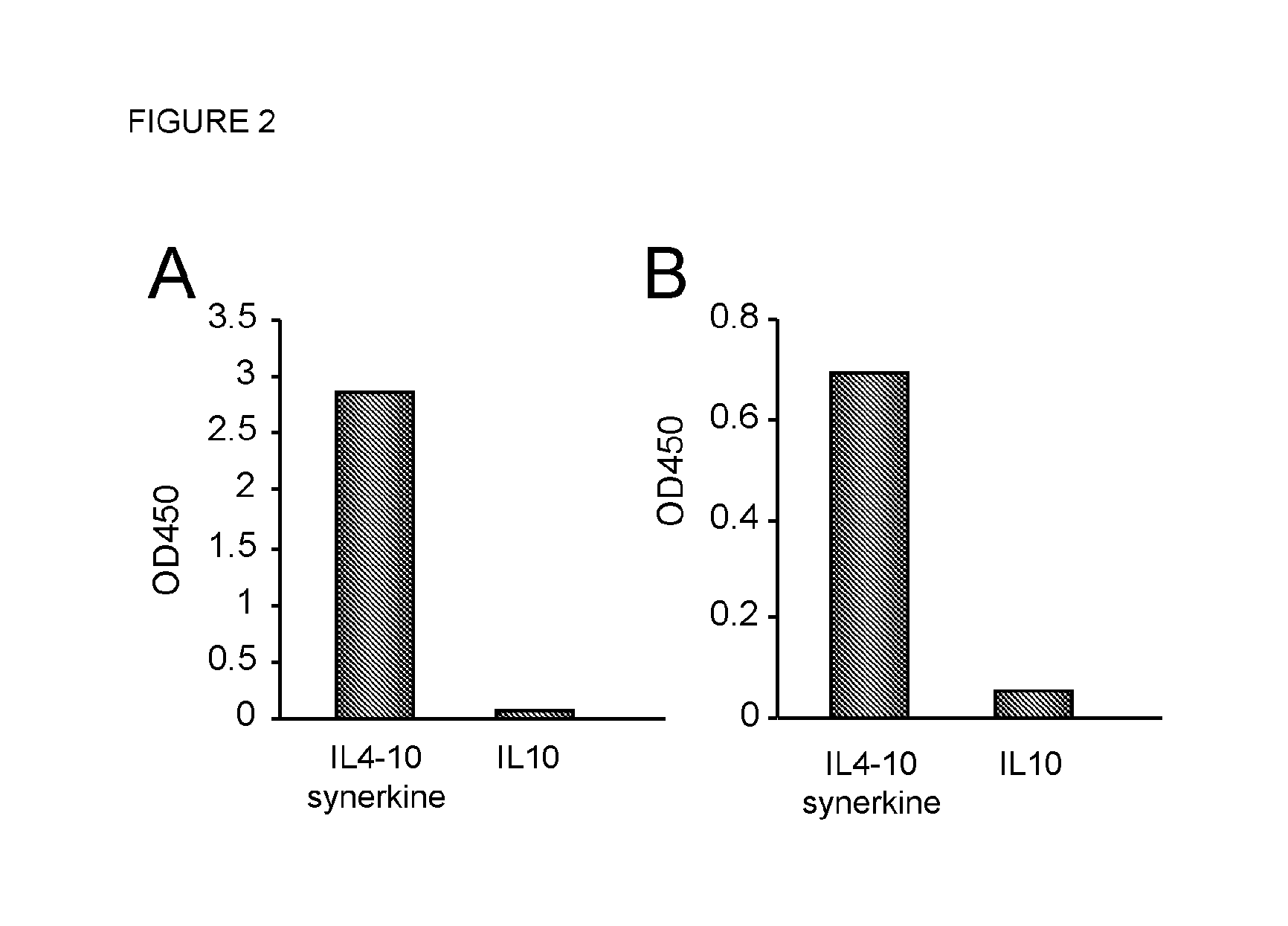
[0126]ELISA Assays for Immunochemical Detection of IL4-IL10 Fusion Protein, IL4 and IL10
[0127]Method:
[0128]The IL4 and IL10 content in culture supernatant or chromatography fractions was measured by ELISA (IL4 PeliPair ELISA Kit; Sanquin, Amsterdam, the Netherlands; Cat# M9314 or IL10 PeliPair ELISA Kit; Sanquin; Cat# M9310) according to manufacturer's instructions. Briefly, catching antibodies against IL4 or IL10 were diluted 1:200 in phosphate buffered saline, pH 7.4 (PBS) and coated overnight onto an ELISA plate. All subsequent steps were performed in PBS supplemented with 0.1%, w / v, Tween-20 (PBS-T). A dose response curve consisting of serial dilutions to yield a range of 100 to 2 pg / ml of recombinant IL4 or IL10 was tested. Bound antibodies were detected with streptavidine-poly-HRP (Sanquin) followed by incubation with TMB (3,3′,5,5″-tetramethylbenzidine; Invitrogen, Carlsbad, Calif., USA; Cat# SB02). Reaction was stopped with 1M Sulphuric Acid (Chem Lab; Cat# CL05-2658-1000). ...
example 3
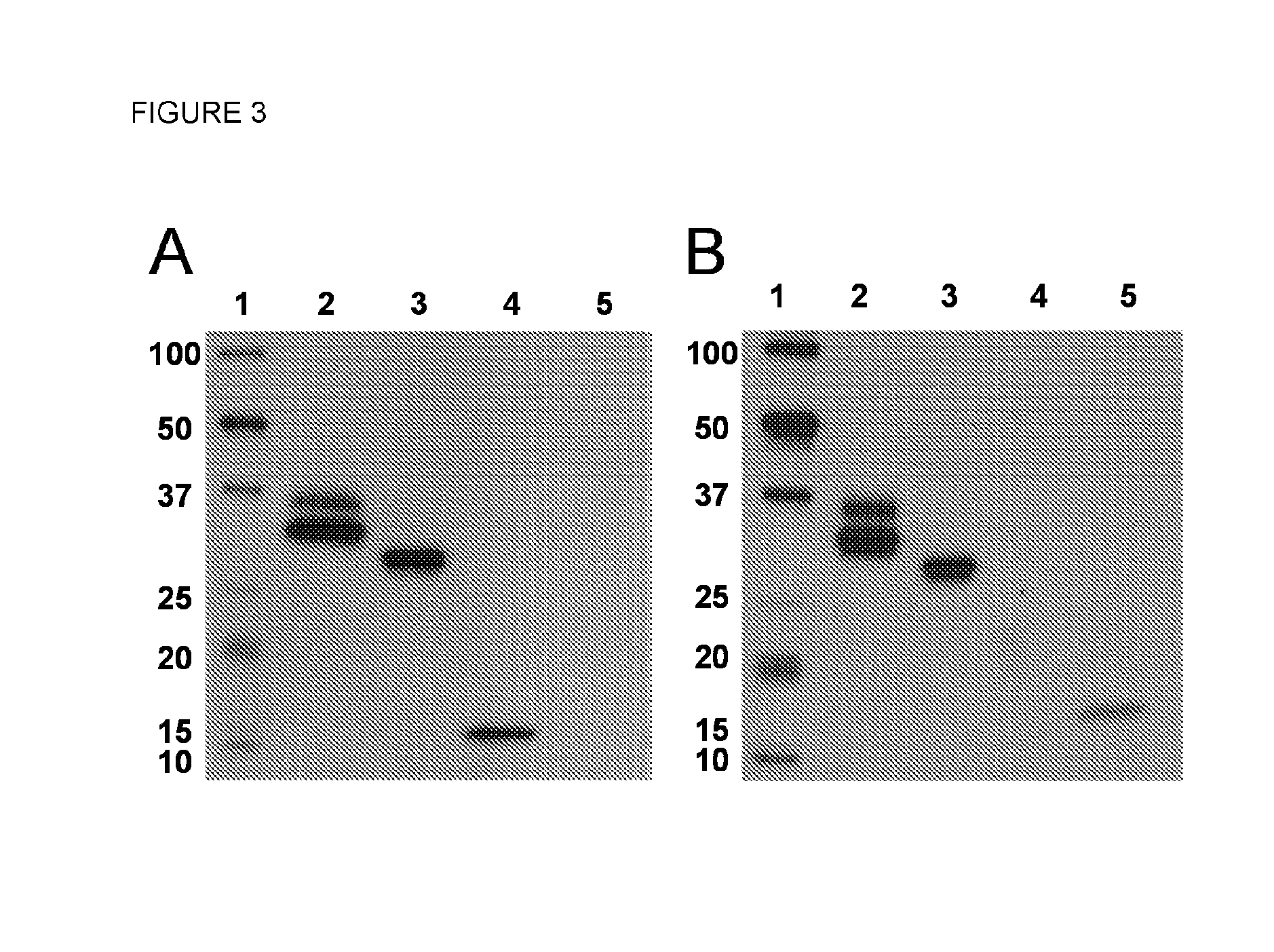
SDS-Page and Western Blotting
[0132]Method:
[0133]Samples were diluted 1:1 in sample buffer (Tris-HCl pH 6.8, 25%, w / v, Glycerol, 2%, w / v, SDS, 0.01%, w / v, bromophenol blue; BioRad, Richmond, Va., USA, Cat#161-0737), containing 710 mM 2-mercaptoethanol and incubated for 10 minutes at 100° C. Subsequently, samples were loaded on a 7.5%, w / v, polyacrylamide Tris / Glycine Gel (Mini-PROTEAN TGX Precast Gels without SDS; BioRad, Cat#456-1023). The molecular weight markers (WesternC Standard, 250-10 kD; BioRad; Cat#161-0376) were run on a separate lane. Electroporesis was performed under reducing conditions, using a Tris / glycine / SDS buffer (BioRad; Cat#161-0732). To identify the IL4-IL10 fusion protein, immunoblotting with anti-IL4 or anti-IL10 antibodies was performed. Proteins were separated on SDS-PAGE as described above, and then transferred to a PVDF-membrane (BioRad; Cat#161-0277) by Western blotting, using a Tris / glycine buffer (BioRad; Cat#161-0734) at 100V for 1 hour.
[0134]After blo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com