Apparatus and method for preparing alcohol from olefin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1.1
Production of Catalyst Solution
[0082]3.2 kg of triphenyl phosphine (TPP) was completely dissolved in 28.7 kg of normal-butyraldehyde having a purity of 99%. 45.9 g of an acetylacetonatodicarbonyl triphenylphosphine rhodium (ROPAC)) catalyst which had been previously weighted was further added to the solution to prepare 32 kg of a catalyst solution.
example 1.2
Preparation of Aldehyde and Gas-Liquid Separation
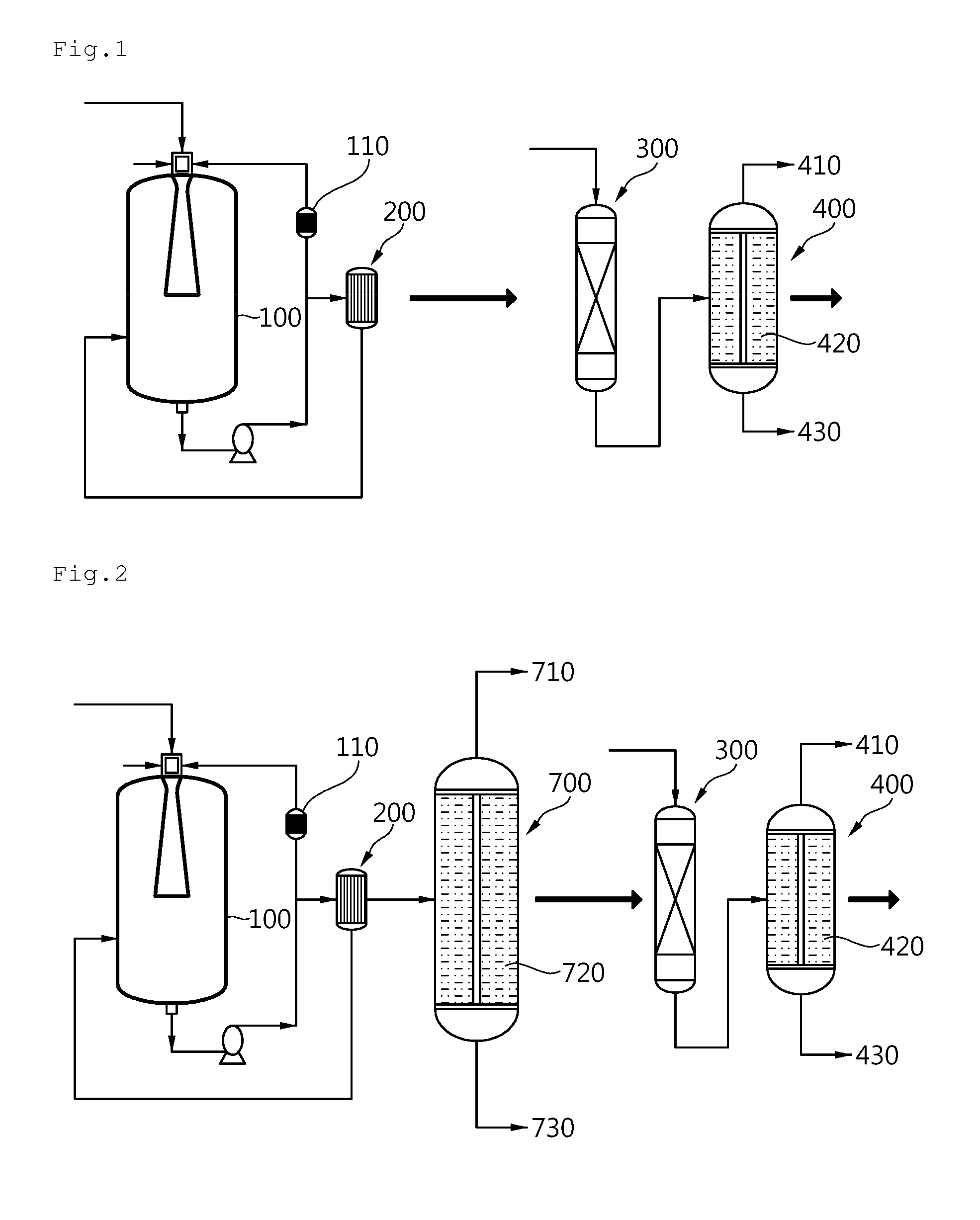
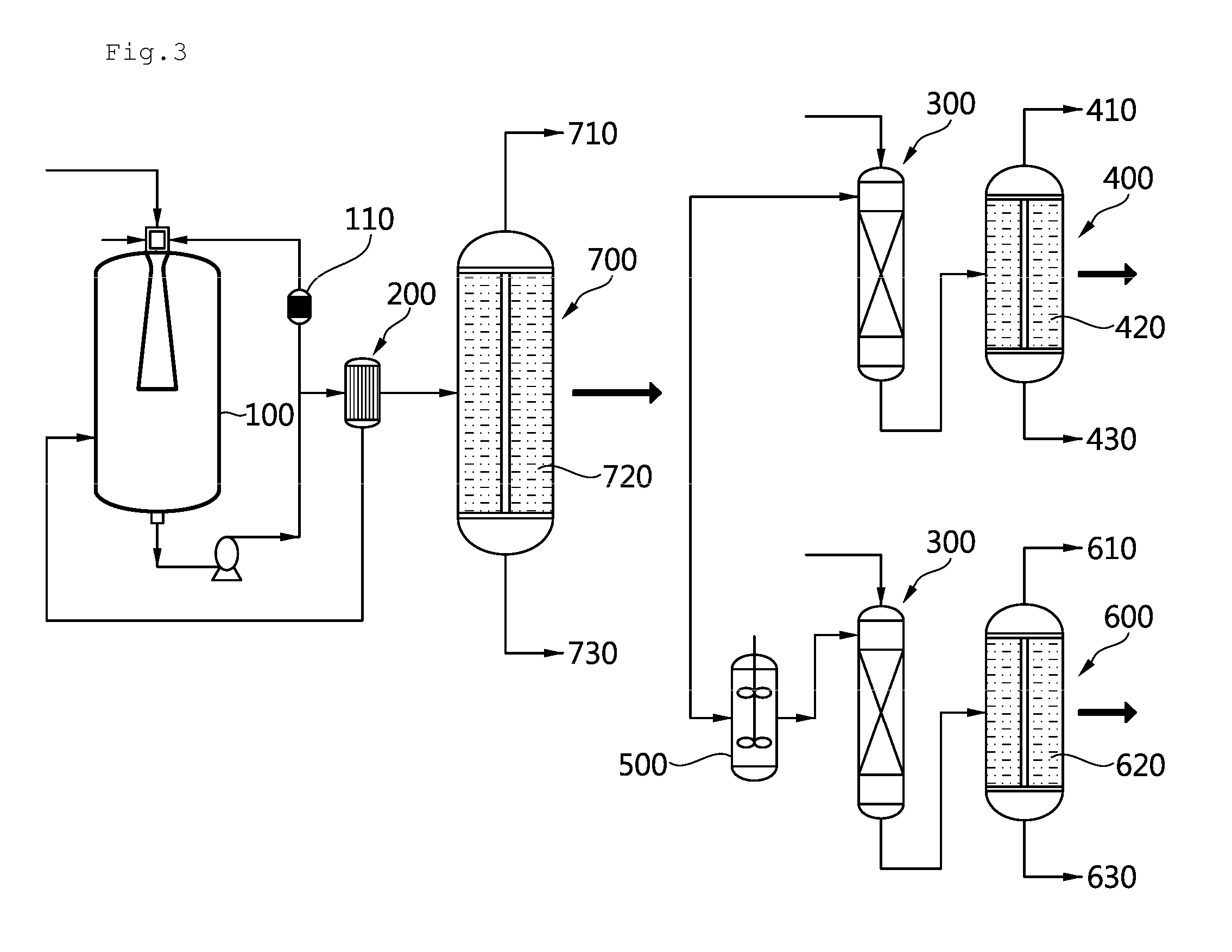
[0083]Referring to FIG. 1, two 30 L loop reactors 100 were produced and a venturi diffusion tube having a nozzle diameter of 5 mm, a diffusion tube inlet diameter of 10 mm, a diffusion tube outlet diameter of 20 mm and a diffusion tube length of 30 mm was mounted on a head of each reactor. In addition, a flat diffusion plate having a diameter of 70 mm was fixed at a position of 200 mm from the lower outlet in the reactor. A circulation pump was mounted in the reactor such that a reaction solution could be recirculated at a flow rate of 20 L / min to a spray nozzle of each reactor head and a heat exchanger 110 was mounted on an external circulation line of both reactors so that reaction heat generated during reaction could be removed.
[0084]The two reactors were connected in series and, for a first reactor, which was a first reactor of the two reactors connected in series, a portion of a circulation line was connected to an upper part of ...
example 1.3
Hydrogenation of Aldehyde Using Fixed Bed Catalyst Reactor
[0090]An alumina ball was charged to a position of 57 cm from an upper part of a column-shaped reactor having a diameter of 8 cm and a length of 330 cm, as a hydrogenation reactor 300, a nickel catalyst supported on gamma-alumina was charged to a length of 315 cm therefrom, and the alumina ball was charged to the remaining lower part thereof. The temperature of the reactor outlet was maintained at a level not higher than 110° C. using an additional circulation pump and an external heat exchanger and an inner pressure of the reactor was maintained at 25 barg.
[0091]Normal-butylalcohol was used as a solvent medium for reaction and heat exchange and a circulation flow was maintained at 38 kg / hr. Upon reaching a normal state, operation was performed for 90 hours and a total weight of a hydrogenation product containing butyl alcohol as a major component was 581 kg and the hydrogenation product was obtained in an amount of 6.45 kg p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com